Induced pluripotent stem cells as a model for diabetes investigation
- PMID: 25716801
- PMCID: PMC4341212
- DOI: 10.1038/srep08597
Induced pluripotent stem cells as a model for diabetes investigation
Abstract
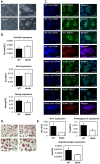
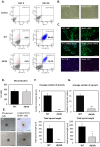
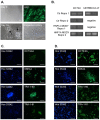
Mouse and human induced pluripotent stem cells (iPSCs) may represent a novel approach for modeling diabetes. Taking this into consideration, the aim of this study was to generate and evaluate differentiation potential of iPSCs from lep(db/db) (db/db) mice, the model of diabetes type 2 as well as from patients with Maturity Onset Diabetes of the Young 3 (HNF1A MODY). Murine iPSC colonies from both wild type and db/db mice were positive for markers of pluripotency: Oct3/4A, Nanog, SSEA1, CDy1 and alkaline phosphatase and differentiated in vitro and in vivo into cells originating from three germ layers. However, our results suggest impaired differentiation of db/db cells into endothelial progenitor-like cells expressing CD34 and Tie2 markers and their reduced angiogenic potential. Human control and HNF1A MODY reprogrammed cells also expressed pluripotency markers: OCT3/4A, SSEA4, TRA-1-60, TRA-1-81, formed embryoid bodies (EBs) and differentiated into cells of three germ layers. Additionally, insulin expressing cells were obtained from those partially reprogrammed cells with direct as well as EB-mediated differentiation method. Our findings indicate that disease-specific iPSCs may help to better understand the mechanisms responsible for defective insulin production or vascular dysfunction upon differentiation toward cell types affected by diabetes.
Figures




References
-
- Danaei G. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378, 31–40 (2011). - PubMed
-
- Kahn C. R. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 43, 1066–1084 (1994). - PubMed
-
- Roberts A. C. & Porter K. E. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diab Vasc Dis Res 10, 472–482 (2013). - PubMed
-
- Chen H. et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84, 491–495 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

