Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke
- PMID: 25716838
- PMCID: PMC4339351
- DOI: 10.1523/JNEUROSCI.2620-14.2015
Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke
Abstract
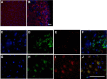
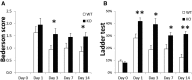
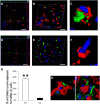
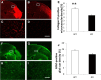
Clearing cellular debris after brain injury represents an important mechanism in regaining tissue homeostasis and promoting functional recovery. Triggering receptor expressed on myeloid cells-2 (TREM2) is a newly identified receptor expressed on microglia and is thought to phagocytose damaged brain cells. The precise role of TREM2 during ischemic stroke has not been fully understood. We explore TREM2 in both in vitro and in vivo stroke models and identify a potential endogenous TREM2 ligand. TREM2 knockdown in microglia reduced microglial activation to an amoeboid phenotype and decreased the phagocytosis of injured neurons. Phagocytosis and infarcted brain tissue resorption was reduced in TREM2 knock-out (KO) mice compared with wild-type (WT) mice. TREM2 KO mice also had worsened neurological recovery and decreased viable brain tissue in the ipsilateral hemisphere. The numbers of activated microglia and phagocytes in TREM2 KO mice were decreased compared with WT mice, and foamy macrophages were nearly absent in the TREM2 KO mice. Postischemia, TREM2 was highly expressed on microglia and TREM2-Fc fusion protein (used as a probe to identify potential TREM2 binding partners) bound to an unknown TREM2 ligand that colocalized to neurons. Oxygen glucose deprivation-exposed neuronal media, or cellular fractions containing nuclei or purified DNA, but not cytosolic fractions, stimulated signaling through TREM2. TREM2-Fc fusion protein pulled down nucleic acids from ischemic brain lysate. These findings establish the relevance of TREM2 in the phagocytosis of the infarcted brain and emphasize its role in influencing neurological outcomes following stroke. Further, nucleic acids may be one potential ligand of TREM2 in brain ischemia.
Keywords: ischemia; neuroprotection; permanent ischemia; phagocytosis; stroke; triggering receptor expressed on myeloid cells-2.
Copyright © 2015 the authors 0270-6474/15/353384-13$15.00/0.
Figures







References
-
- Bianchin MM, Capella HM, Chaves DL, Steindel M, Grisard EC, Ganev GG, da Silva Júnior JP, Neto Evaldo S, Poffo MA, Walz R, Carlotti Júnior CG, Sakamoto AC. Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy–PLOSL): a dementia associated with bone cystic lesions. From clinical to genetic and molecular aspects. Cell Mol Neurobiol. 2004;24:1–24. doi: 10.1023/B:CEMN.0000012721.08168.ee. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
