Parkinson's disease iron deposition caused by nitric oxide-induced loss of β-amyloid precursor protein
- PMID: 25716857
- PMCID: PMC6605561
- DOI: 10.1523/JNEUROSCI.3439-14.2015
Parkinson's disease iron deposition caused by nitric oxide-induced loss of β-amyloid precursor protein
Abstract
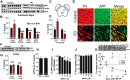
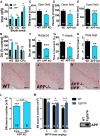
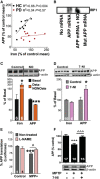
Elevation of both neuronal iron and nitric oxide (NO) in the substantia nigra are associated with Parkinson's disease (PD) pathogenesis. We reported previously that the Alzheimer-associated β-amyloid precursor protein (APP) facilitates neuronal iron export. Here we report markedly decreased APP expression in dopaminergic neurons of human PD nigra and that APP(-/-) mice develop iron-dependent nigral cell loss. Conversely, APP-overexpressing mice are protected in the MPTP PD model. NO suppresses APP translation in mouse MPTP models, explaining how elevated NO causes iron-dependent neurodegeneration in PD.
Keywords: APP; iron; nitric oxide.
Copyright © 2015 the authors 0270-6474/15/353591-07$15.00/0.
Figures

References
-
- Ayton S, Lei P, Duce JA, Wong BX, Sedjahtera A, Adlard PA, Bush AI, Finkelstein DI. Ceruloplasmin dysfunction and therapeutic potential for parkinson disease. Ann Neurol. 2013;73:554–559. - PubMed
-
- Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F, Baru V, Lou Y, Freyzon Y, Cho S, Mungenast AE, Muffat J, Mitalipova M, Pluth MD, Jui NT, Schüle B, Lippard SJ, Tsai LH, Krainc D, Buchwald SL, Jaenisch R, Lindquist S. Identification and rescue of alpha-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. - DOI - PMC - PubMed
-
- Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G, Garçon G, Rouaix N, Duhamel A, Jissendi P, Dujardin K, Auger F, Ravasi L, Hopes L, Grolez G, Firdaus W, Sablonnière B, Strubi-Vuillaume I, Zahr N, Destée A, Corvol JC, Pöltl D, Leist M, Rose C, Defebvre L, Marchetti P, Cabantchik ZI, Bordet R. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxid Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
