Anti-muscarinic adjunct therapy accelerates functional human oligodendrocyte repair
- PMID: 25716865
- PMCID: PMC4464275
- DOI: 10.1523/JNEUROSCI.3510-14.2015
Anti-muscarinic adjunct therapy accelerates functional human oligodendrocyte repair
Abstract
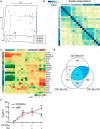
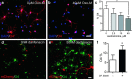
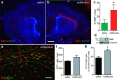
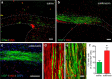
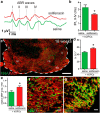
Therapeutic repair of myelin disorders may be limited by the relatively slow rate of human oligodendrocyte differentiation. To identify appropriate pharmacological targets with which to accelerate differentiation of human oligodendrocyte progenitors (hOPCs) directly, we used CD140a/O4-based FACS of human forebrain and microarray to hOPC-specific receptors. Among these, we identified CHRM3, a M3R muscarinic acetylcholine receptor, as being restricted to oligodendrocyte-biased CD140a(+)O4(+) cells. Muscarinic agonist treatment of hOPCs resulted in a specific and dose-dependent blockade of oligodendrocyte commitment. Conversely, when hOPCs were cocultured with human neurons, M3R antagonist treatment stimulated oligodendrocytic differentiation. Systemic treatment with solifenacin, an FDA-approved muscarinic receptor antagonist, increased oligodendrocyte differentiation of transplanted hOPCs in hypomyelinated shiverer/rag2 brain. Importantly, solifenacin treatment of engrafted animals reduced auditory brainstem response interpeak latency, indicative of increased conduction velocity and thereby enhanced functional repair. Therefore, solifenacin and other selective muscarinic antagonists represent new adjunct approaches to accelerate repair by engrafted human progenitors.
Keywords: glia; human; microarray; oligodendrocyte.
Copyright © 2015 the authors 0270-6474/15/353676-13$15.00/0.
Figures

References
-
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
-
- Brand-Schieber E, Werner P. (+/-)-Alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and kainate receptor subunit expression in mouse versus rat spinal cord white matter: similarities in astrocytes but differences in oligodendrocytes. Neurosci Lett. 2003;345:126–130. doi: 10.1016/S0304-3940(03)00469-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
