An active role for basement membrane assembly and modification in tissue sculpting
- PMID: 25717004
- PMCID: PMC4446735
- DOI: 10.1242/jcs.168021
An active role for basement membrane assembly and modification in tissue sculpting
Abstract
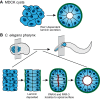
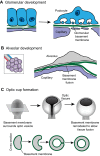
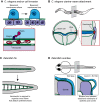
Basement membranes are a dense, sheet-like form of extracellular matrix (ECM) that underlie epithelia and endothelia, and surround muscle, fat and Schwann cells. Basement membranes separate tissues and protect them from mechanical stress. Although traditionally thought of as a static support structure, a growing body of evidence suggests that dynamic basement membrane deposition and modification instructs coordinated cellular behaviors and acts mechanically to sculpt tissues. In this Commentary, we highlight recent studies that support the idea that far from being a passive matrix, basement membranes play formative roles in shaping tissues.
© 2015. Published by The Company of Biologists Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

