Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study
- PMID: 25717116
- PMCID: PMC4399443
- DOI: 10.1093/asj/sju084
Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study
Erratum in
-
Corrigendum on: ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study.Aesthet Surg J. 2015 Nov;35(8):1044. doi: 10.1093/asj/sjv172. Aesthet Surg J. 2015. PMID: 26508651 Free PMC article. No abstract available.
Abstract
Background: Silicone breast implants have long been used for breast augmentation and reconstruction. During this time, these medical devices have gone through a number of modifications to improve their safety, quality, and clinical outcome performance.
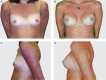
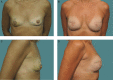
Objectives: The authors conducted a 10-year study to determine the safety and effectiveness of Natrelle 410 silicone breast implants.
Methods: This prospective, multicenter study enrolled 941 subjects who were undergoing either augmentation, augmentation revision, reconstruction, or reconstruction revision. Data on complications, reoperations, explantations, and subject satisfaction were collected at annual clinic visits, and one-third of subjects underwent biennial magnetic resonance imaging (MRI) to screen for implant rupture. The authors used the Kaplan-Meier estimator to calculate risk rates for local complications, reoperations, and explantations.
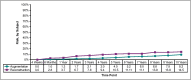
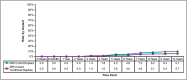
Results: Capsular contracture rates increased approximately 1% per year from the previously reported 6-year rates. The rates were significantly lower than those from the Natrelle round gel core study. The overall rate of confirmed ruptured implants in subjects who underwent MRI was 5.7%. Eleven late seromas were reported. The most common reason for explantation was a subject requesting a size or style change. Satisfaction rates remained high through 10 years, with most subjects saying they were somewhat or definitely satisfied with their implants.
Conclusions: This 10-year prospective trial demonstrated the long-term safety and effectiveness of Natrelle 410 anatomical form-stable implants. The complication rates were low and the satisfaction rates were high. LEVEL OF EVIDENCE 1: Therapeutic.
© 2015 The American Society for Aesthetic Plastic Surgery, Inc. Reprints and permission: journals.permissions@oup.com.
Figures

Comment in
-
Comments on "ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study" and its assigned level of evidence.Aesthet Surg J. 2015 Nov;35(8):NP269. doi: 10.1093/asj/sjv118. Aesthet Surg J. 2015. PMID: 26508653 No abstract available.
References
-
- Jewell ML. Silicone gel breast implants at 50: the state of the science. Aesthet Surg J. 2012;32(8):1031–1034. - PubMed
-
- Bengtson BP, Van Natta BW, Murphy DK, Slicton A, Maxwell GP for the Style 410 U.S. Core Clinical Study Group. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):40S–48S. - PubMed
-
- Maxwell GP, Van Natta BW, Murphy DK, Slicton A, Bengtson BP. Natrelle Style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32(6):709–717. - PubMed
-
- Gladfelter J, Murphy D. Breast augmentation motivations and satisfaction: a prospective study of more than 3,000 silicone implantations. Plast Surg Nurs. 2008;28(4):170–174. quiz 175-176. - PubMed
-
- Maxwell GP, Hartley W. Breast augmentation. In: Mathes SJ, Hentz VR, editors. Plastic Surgery. 2nd ed. Amsterdam: Elsevier; 2006. pp. 1–33.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

