Dissociable effects of local inhibitory and excitatory theta-burst stimulation on large-scale brain dynamics
- PMID: 25717162
- PMCID: PMC4443609
- DOI: 10.1152/jn.00850.2014
Dissociable effects of local inhibitory and excitatory theta-burst stimulation on large-scale brain dynamics
Abstract
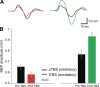
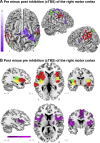
Normal brain function depends on a dynamic balance between local specialization and large-scale integration. It remains unclear, however, how local changes in functionally specialized areas can influence integrated activity across larger brain networks. By combining transcranial magnetic stimulation with resting-state functional magnetic resonance imaging, we tested for changes in large-scale integration following the application of excitatory or inhibitory stimulation on the human motor cortex. After local inhibitory stimulation, regions encompassing the sensorimotor module concurrently increased their internal integration and decreased their communication with other modules of the brain. There were no such changes in modular dynamics following excitatory stimulation of the same area of motor cortex nor were there changes in the configuration and interactions between core brain hubs after excitatory or inhibitory stimulation of the same area. These results suggest the existence of selective mechanisms that integrate local changes in neural activity, while preserving ongoing communication between brain hubs.
Keywords: TMS; brain network; connectivity; fMRI; hubs; modularity.
Copyright © 2015 the American Physiological Society.
Figures





References
-
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19: 1950–1962, 2004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

