The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy
- PMID: 25717303
- PMCID: PMC4324070
- DOI: 10.3389/fphys.2015.00022
The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy
Abstract
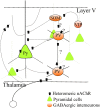
Autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) is a focal epilepsy with attacks typically arising in the frontal lobe during non-rapid eye movement (NREM) sleep. It is characterized by clusters of complex and stereotyped hypermotor seizures, frequently accompanied by sudden arousals. Cognitive and psychiatric symptoms may be also observed. Approximately 12% of the ADNFLE families carry mutations on genes coding for subunits of the heteromeric neuronal nicotinic receptors (nAChRs). This is consistent with the widespread expression of these receptors, particularly the α4β2(*) subtype, in the neocortex and thalamus. However, understanding how mutant nAChRs lead to partial frontal epilepsy is far from being straightforward because of the complexity of the cholinergic regulation in both developing and mature brains. The relation with the sleep-waking cycle must be also explained. We discuss some possible pathogenetic mechanisms in the light of recent advances about the nAChR role in prefrontal regions as well as the studies carried out in murine models of ADNFLE. Functional evidence points to alterations in prefrontal GABA release, and the synaptic unbalance probably arises during the cortical circuit maturation. Although most of the available functional evidence concerns mutations on nAChR subunit genes, other genes have been recently implicated in the disease, such as KCNT1 (coding for a Na(+)-dependent K(+) channel), DEPD5 (Disheveled, Egl-10 and Pleckstrin Domain-containing protein 5), and CRH (Corticotropin-Releasing Hormone). Overall, the uncertainties about both the etiology and the pathogenesis of ADNFLE point to the current gaps in our knowledge the regulation of neuronal networks in the cerebral cortex.
Keywords: ADNFLE; CHRNA2; CHRNA4; CHRNB2; GABA; nAChR; prefrontal cortex; sleep-related epilepsy.
Figures
References
-
- Amzica F., Steriade M. (2002). Cellular mechanisms underlying seizure activity during sleep, in Sleep and Epilepsy: the Clinical Spectrum, eds Bazil C. W., Malow B. A., Sammaritano M. R. (Amsterdam: Elsevier Science; ), 109–126.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

