Adoptive T cell immunotherapy for cancer
- PMID: 25717386
- PMCID: PMC4327320
- DOI: 10.5041/RMMJ.10179
Adoptive T cell immunotherapy for cancer
Abstract
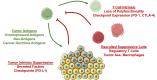
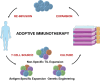
Harnessing the immune system to recognize and destroy tumor cells has been the central goal of anti-cancer immunotherapy. In recent years, there has been an increased interest in optimizing this technology in order to make it a clinically feasible treatment. One of the main treatment modalities within cancer immunotherapy has been adoptive T cell therapy (ACT). Using this approach, tumor-specific cytotoxic T cells are infused into cancer patients with the goal of recognizing, targeting, and destroying tumor cells. In the current review, we revisit some of the major successes of ACT, the major hurdles that have been overcome to optimize ACT, the remaining challenges, and future approaches to make ACT widely available.
Keywords: Adoptive T cell transfer; T cell engineering; cancer immunotherapy.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

