Aberrant lymphatic endothelial progenitors in lymphatic malformation development
- PMID: 25719418
- PMCID: PMC4342011
- DOI: 10.1371/journal.pone.0117352
Aberrant lymphatic endothelial progenitors in lymphatic malformation development
Abstract
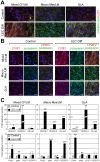
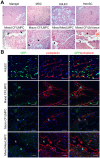
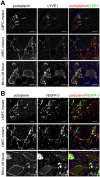
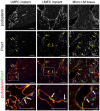
Lymphatic malformations (LMs) are vascular anomalies thought to arise from dysregulated lymphangiogenesis. These lesions impose a significant burden of disease on affected individuals. LM pathobiology is poorly understood, hindering the development of effective treatments. In the present studies, immunostaining of LM tissues revealed that endothelial cells lining aberrant lymphatic vessels and cells in the surrounding stroma expressed the stem cell marker, CD133, and the lymphatic endothelial protein, podoplanin. Isolated patient-derived CD133+ LM cells expressed stem cell genes (NANOG, Oct4), circulating endothelial cell precursor proteins (CD90, CD146, c-Kit, VEGFR-2), and lymphatic endothelial proteins (podoplanin, VEGFR-3). Consistent with a progenitor cell identity, CD133+ LM cells were multipotent and could be differentiated into fat, bone, smooth muscle, and lymphatic endothelial cells in vitro. CD133+ cells were compared to CD133- cells isolated from LM fluids. CD133- LM cells had lower expression of stem cell genes, but expressed circulating endothelial precursor proteins and high levels of lymphatic endothelial proteins, VE-cadherin, CD31, podoplanin, VEGFR-3 and Prox1. CD133- LM cells were not multipotent, consistent with a differentiated lymphatic endothelial cell phenotype. In a mouse xenograft model, CD133+ LM cells differentiated into lymphatic endothelial cells that formed irregularly dilated lymphatic channels, phenocopying human LMs. In vivo, CD133+ LM cells acquired expression of differentiated lymphatic endothelial cell proteins, podoplanin, LYVE1, Prox1, and VEGFR-3, comparable to expression found in LM patient tissues. Taken together, these data identify a novel LM progenitor cell population that differentiates to form the abnormal lymphatic structures characteristic of these lesions, recapitulating the human LM phenotype. This LM progenitor cell population may contribute to the clinically refractory behavior of LMs.
Conflict of interest statement
Figures




References
-
- Mulliken JB, Fishman SJ, Burrows PE (2000) Vascular anomalies. Current problems in surgery 37: 517–584. - PubMed
-
- Mulliken JB, Glowacki J (1982) Classification of pediatric vascular lesions. Plastic and reconstructive surgery 70: 120–121. - PubMed
-
- Mulliken JB, Glowacki J (1982) Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plastic and reconstructive surgery 69: 412–422. - PubMed
-
- Raveh E, de Jong AL, Taylor GP, Forte V (1997) Prognostic factors in the treatment of lymphatic malformations. Archives of otolaryngology—head & neck surgery 123: 1061–1065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

