Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND)
- PMID: 25719800
- PMCID: PMC4342256
- DOI: 10.1371/journal.pone.0116526
Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND)
Abstract
Background: HIV associated neurocognitive disorders (HAND) continue to affect cognition and everyday functioning despite anti-retroviral treatment (ART). Previous studies focused on mechanisms related to monocyte/macrophage mediated inflammation. However, in the ART era, there is increasing evidence for the involvement of CD8+ T-cells in CNS pathogenesis.
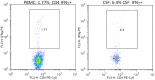
Methods: To investigate the relationship between T-cell responses and neurocognitive impairment (NCI), cerebrospinal fluid (CSF) and peripheral blood CD4+ and CD8+ T-cell intracellular cytokine (IFNγ, IL-2, TNFα) and lytic marker (CD107a) expression were assessed in HIV infected subjects who underwent comprehensive neurocognitive (NC) evaluation and either initiated or changed ART.
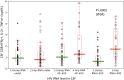
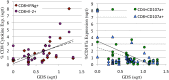
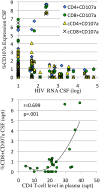
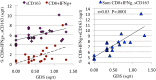
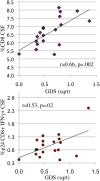
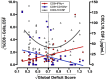
Results: Data were collected from 31 participants at 70 visits. The frequency of cytokine expressing T-cells in CSF was significantly higher than in peripheral blood for CD4+T-cells: TNFα, IL-2, IFNγ and CD8+T-cells: IL-2 and IFNγ. Analysis of T-cell activity and NCI as a function of CSF HIV RNA levels suggested a general association between NCI, high CSF CD8+ (but not CD4+T-cell) cytokine expression and CSF HIV RNA <103 copies/ml (p<0.0001). Specifically, CSF CD8+ T-cell IFNγ expression correlated with severity of NCI (r = 0.57, p = 0.004). Multivariable analyses indicated that CSF CD8+T-cell IFNγ and myeloid activation (CD163) contributed equally and independently to cognitive status and a composite variable produced the strongest correlation with NCI (r = 0.83, p = 0.0001). In contrast, CD8+ cytolytic activity (CD107a expression) was negatively correlated with NCI (p = 0.05) but was dependent on CD4 levels >400/μl and low CSF HIV RNA levels (<103 copies/ml). In our longitudinal analysis of 16 subjects, higher CSF CD8+IFNγ expression at baseline predicted NC decline at follow-up (p = 0.02). Severity of NCI at follow-up correlated with level of residual HIV RNA in CSF.
Conclusions: Presence of IFNγ expressing CD8+ T-cells, absence of cytolytic CD8+ T-cells, high myeloid activation, and failure of ART to suppress HIV replication in CSF contribute to increased risk of HAND.
Conflict of interest statement
Figures
References
-
- Masliah E, Ge N, Mucke L (1996) Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol 10: 57–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K24 MH097673/MH/NIMH NIH HHS/United States
- P50 DA026306/DA/NIDA NIH HHS/United States
- P30 MH62512/MH/NIMH NIH HHS/United States
- DA26306/DA/NIDA NIH HHS/United States
- R01 MH058076/MH/NIMH NIH HHS/United States
- R01 HL090975/HL/NHLBI NIH HHS/United States
- T32 AI007384/AI/NIAID NIH HHS/United States
- P30 MH062512/MH/NIMH NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- R21 MH85610/MH/NIMH NIH HHS/United States
- U19 AI096113/AI/NIAID NIH HHS/United States
- R21 MH085610/MH/NIMH NIH HHS/United States
- R01 MH58076/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

