Material properties of biofilms-a review of methods for understanding permeability and mechanics
- PMID: 25719969
- PMCID: PMC4504244
- DOI: 10.1088/0034-4885/78/3/036601
Material properties of biofilms-a review of methods for understanding permeability and mechanics
Abstract
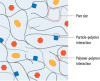
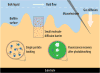
Microorganisms can form biofilms, which are multicellular communities surrounded by a hydrated extracellular matrix of polymers. Central properties of the biofilm are governed by this extracellular matrix, which provides mechanical stability to the 3D biofilm structure, regulates the ability of the biofilm to adhere to surfaces, and determines the ability of the biofilm to adsorb gases, solutes, and foreign cells. Despite their critical relevance for understanding and eliminating of biofilms, the materials properties of the extracellular matrix are understudied. Here, we offer the reader a guide to current technologies that can be utilized to specifically assess the permeability and mechanical properties of the biofilm matrix and its interacting components. In particular, we highlight technological advances in instrumentation and interactions between multiple disciplines that have broadened the spectrum of methods available to conduct these studies. We review pioneering work that furthers our understanding of the material properties of biofilms.
Figures


References
-
- AGGARWAL S, HOZALSKI RM. Determination of biofilm mechanical properties from tensile tests performed using a micro-cantilever method. Biofouling. 2010;26:479–486. - PubMed
-
- AGGARWAL S, POPPELE EH, HOZALSKI RM. Development and testing of a novel microcantilever technique for measuring the cohesive strength of intact biofilms. Biotechnology and Bioengineering. 2010;105:924–934. - PubMed
-
- AMSDEN B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules. 1998;31:8382–8395.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
