On the lag phase in amyloid fibril formation
- PMID: 25719972
- PMCID: PMC4498454
- DOI: 10.1039/c4cp05563b
On the lag phase in amyloid fibril formation
Abstract
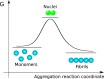
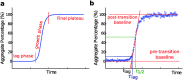
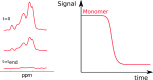
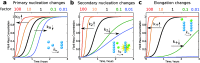
The formation of nanoscale amyloid fibrils from normally soluble peptides and proteins is a common form of self-assembly phenomenon that has fundamental connections with biological functions and human diseases. The kinetics of this process has been widely studied and exhibits on a macroscopic level three characteristic stages: a lag phase, a growth phase and a final plateau regime. The question of which molecular events take place during each one of these phases has been a central element in the quest for a mechanism of amyloid formation. In this review, we discuss the nature and molecular origin of the lag-phase in amyloid formation by making use of tools and concepts from physical chemistry, in particular from chemical reaction kinetics. We discuss how, in macroscopic samples, it has become apparent that the lag-phase is not a waiting time for nuclei to form. Rather, multiple parallel processes exist and typically millions of primary nuclei form during the lag phase from monomers in solution. Thus, the lag-time represents a time that is required for the nuclei that are formed early on in the reaction to grow and proliferate in order to reach an aggregate concentration that is readily detected in bulk assays. In many cases, this proliferation takes place through secondary nucleation, where fibrils may present a catalytic surface for the formation of new aggregates. Fibrils may also break (fragmentation) and thereby provide new ends for elongation. Thus, at least two - primary nucleation and elongation - and in many systems at least four - primary nucleation, elongation, secondary nucleation and fragmentation - microscopic processes occur during the lag phase. Moreover, these same processes occur during all three phases of the macroscopic aggregation process, albeit at different rates as governed by rate constants and by the concentration of reacting species at each point in time.
Figures

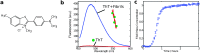
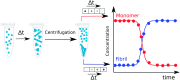
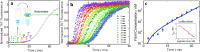


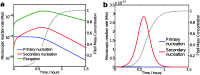



References
-
- Sipe J. D., Benson M. D., Buxbaum J. N., Ikeda S.-I., Merlini G., Saraiva M. J. M., Westermark P. Amyloid. 2012;19:167–170. - PubMed
-
- Jarrett J. T., Lansbury P. T. Cell. 1993;73:1055–1058. - PubMed
-
- Lansbury P. T., Lashuel H. A. Nature. 2006;443:774–779. - PubMed
-
- Chiti F. and Dobson C. M., Annual Review of Biochemistry, 2006, vol. 75, pp. 333–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

