Repeated intravitreous ranibizumab injections for diabetic macular edema and the risk of sustained elevation of intraocular pressure or the need for ocular hypotensive treatment
- PMID: 25719991
- PMCID: PMC4496789
- DOI: 10.1001/jamaophthalmol.2015.186
Repeated intravitreous ranibizumab injections for diabetic macular edema and the risk of sustained elevation of intraocular pressure or the need for ocular hypotensive treatment
Abstract
Importance: For the management of retinal disease, the use of intravitreous injections of anti-vascular endothelial growth factor has increased. Recent reports have suggested that this therapy may cause sustained elevation of intraocular pressure (IOP) and may potentially increase the risk of glaucoma for patients with retinal disease.
Objective: To assess the risk of sustained IOP elevation or the need for IOP-lowering treatments for eyes with diabetic macular edema following repeated intravitreous injections of ranibizumab.
Design, setting, and participants: An exploratory analysis was conducted within a Diabetic Retinopathy Clinical Research Network randomized clinical trial. Study enrollment dates were from March 20, 2007, to December 17, 2008. Of 582 eyes (of 486 participants) with center-involved diabetic macular edema and no preexisting open-angle glaucoma, 260 were randomly assigned to receive a sham injection plus focal/grid laser treatment, and 322 were randomly assigned to receive ranibizumab plus deferred or prompt focal/grid laser treatment.
Main outcomes and measures: The cumulative probability of sustained IOP elevation, defined as IOP of at least 22 mm Hg and an increase of at least 6 mm Hg from baseline at 2 consecutive visits, or the initiation or augmentation of ocular hypotensive therapy, through 3 years of follow-up.
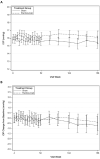
Results: The mean (SD) baseline IOP in both treatment groups was 16 (3) mm Hg (range, 5-24 mm Hg). The cumulative probability of sustained IOP elevation or of initiation or augmentation of ocular hypotensive therapy by 3 years, after repeated ranibizumab injections, was 9.5% for the participants who received ranibizumab plus prompt or deferred focal/grid laser treatment vs 3.4% for the participants who received a sham injection plus focal/grid laser treatment (difference, 6.1% [99% CI, -0.2% to 12.3%]; hazard ratio, 2.9 [99% CI, 1.0-7.9]; P = .01). The distribution of IOP and the change in IOP from baseline at each visit through 3 years were similar in each group.
Conclusions and relevance: In eyes with center-involved diabetic macular edema and no prior open-angle glaucoma, repeated intravitreous injections of ranibizumab may increase the risk of sustained IOP elevation or the need for ocular hypotensive treatment. Clinicians should be aware of this risk and should consider this information when following up with patients who have received intravitreous injections of anti-vascular endothelial growth factor for the treatment of diabetic macular edema.
Figures

References
-
- Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. - PubMed
-
- Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–7. - PubMed
-
- Bakri SJ, Pulido JS, McCannel CA, Hodge DO, Diehl N, Hillemeier J. Immediate intraocular pressure changes following intravitreal injections of triamcinolone, pegaptanib, and bevacizumab. Eye (Lond) 2009;23:181–5. - PubMed
-
- Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011;95:1111–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

