Epigenetic Regulation of Placenta-Specific 8 Contributes to Altered Function of Endothelial Colony-Forming Cells Exposed to Intrauterine Gestational Diabetes Mellitus
- PMID: 25720387
- PMCID: PMC4477353
- DOI: 10.2337/db14-1709
Epigenetic Regulation of Placenta-Specific 8 Contributes to Altered Function of Endothelial Colony-Forming Cells Exposed to Intrauterine Gestational Diabetes Mellitus
Abstract
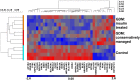
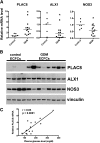
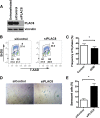
Intrauterine exposure to gestational diabetes mellitus (GDM) is linked to development of hypertension, obesity, and type 2 diabetes in children. Our previous studies determined that endothelial colony-forming cells (ECFCs) from neonates exposed to GDM exhibit impaired function. The current goals were to identify aberrantly expressed genes that contribute to impaired function of GDM-exposed ECFCs and to evaluate for evidence of altered epigenetic regulation of gene expression. Genome-wide mRNA expression analysis was conducted on ECFCs from control and GDM pregnancies. Candidate genes were validated by quantitative RT-PCR and Western blotting. Bisulfite sequencing evaluated DNA methylation of placenta-specific 8 (PLAC8). Proliferation and senescence assays of ECFCs transfected with siRNA to knockdown PLAC8 were performed to determine functional impact. Thirty-eight genes were differentially expressed between control and GDM-exposed ECFCs. PLAC8 was highly expressed in GDM-exposed ECFCs, and PLAC8 expression correlated with maternal hyperglycemia. Methylation status of 17 CpG sites in PLAC8 negatively correlated with mRNA expression. Knockdown of PLAC8 in GDM-exposed ECFCs improved proliferation and senescence defects. This study provides strong evidence in neonatal endothelial progenitor cells that GDM exposure in utero leads to altered gene expression and DNA methylation, suggesting the possibility of altered epigenetic regulation.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

Similar articles
-
Gestational diabetes induces alterations in the function of neonatal endothelial colony-forming cells.Pediatr Res. 2014 Feb;75(2):266-72. doi: 10.1038/pr.2013.224. Epub 2013 Nov 14. Pediatr Res. 2014. PMID: 24232636 Free PMC article.
-
Human fetoplacental arterial and venous endothelial cells are differentially programmed by gestational diabetes mellitus, resulting in cell-specific barrier function changes.Diabetologia. 2018 Nov;61(11):2398-2411. doi: 10.1007/s00125-018-4699-7. Epub 2018 Aug 8. Diabetologia. 2018. PMID: 30091044 Free PMC article.
-
Vitamin D rescues dysfunction of fetal endothelial colony forming cells from individuals with gestational diabetes.Placenta. 2015 Apr;36(4):410-8. doi: 10.1016/j.placenta.2015.01.195. Epub 2015 Feb 7. Placenta. 2015. PMID: 25684656
-
Gestational diabetes mellitus, programing and epigenetics.J Matern Fetal Neonatal Med. 2014 Aug;27(12):1266-9. doi: 10.3109/14767058.2013.853733. Epub 2013 Oct 30. J Matern Fetal Neonatal Med. 2014. PMID: 24125565 Review.
-
Role of insulin and adenosine in the human placenta microvascular and macrovascular endothelial cell dysfunction in gestational diabetes mellitus.Microcirculation. 2014 Jan;21(1):26-37. doi: 10.1111/micc.12077. Microcirculation. 2014. PMID: 23875992 Review.
Cited by
-
Integrated bioinformatics analysis reveals novel key biomarkers in diabetic nephropathy.SAGE Open Med. 2022 Nov 11;10:20503121221137005. doi: 10.1177/20503121221137005. eCollection 2022. SAGE Open Med. 2022. PMID: 36385790 Free PMC article.
-
A Cell-Based Approach to Study the Associations Between Mitochondrial Health, Early Life Exposures, and Consequent Health Outcomes.Front Public Health. 2019 Mar 13;7:36. doi: 10.3389/fpubh.2019.00036. eCollection 2019. Front Public Health. 2019. PMID: 30918888 Free PMC article. No abstract available.
-
PLAC8-Mediated Activation of NOX4 Signalling Restores Angiogenic Function of Endothelial Colony-Forming Cells in Experimental Hypoxia.Cells. 2023 Sep 6;12(18):2220. doi: 10.3390/cells12182220. Cells. 2023. PMID: 37759443 Free PMC article.
-
PLAC8 Expression Regulates Trophoblast Invasion and Conversion into an Endothelial Phenotype (eEVT).Int J Mol Sci. 2025 Jun 4;26(11):5371. doi: 10.3390/ijms26115371. Int J Mol Sci. 2025. PMID: 40508180 Free PMC article.
-
Decreased Lymphangiogenic Activities and Genes Expression of Cord Blood Lymphatic Endothelial Progenitor Cells (VEGFR3+/Pod+/CD11b+ Cells) in Patient with Preeclampsia.Int J Mol Sci. 2021 Apr 19;22(8):4237. doi: 10.3390/ijms22084237. Int J Mol Sci. 2021. PMID: 33921847 Free PMC article.
References
-
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989;2:577–580 - PubMed
-
- Cho NH, Silverman BL, Rizzo TA, Metzger BE. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J Pediatr 2000;136:587–592 - PubMed
-
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–e296 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

