Exenatide Protects Against Glucose- and Lipid-Induced Endothelial Dysfunction: Evidence for Direct Vasodilation Effect of GLP-1 Receptor Agonists in Humans
- PMID: 25720388
- PMCID: PMC4477348
- DOI: 10.2337/db14-0976
Exenatide Protects Against Glucose- and Lipid-Induced Endothelial Dysfunction: Evidence for Direct Vasodilation Effect of GLP-1 Receptor Agonists in Humans
Abstract
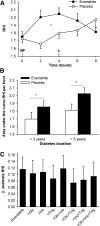
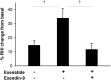
GLP-1 receptor (GLP-1R) agonists may improve endothelial function (EF) via metabolic improvement and direct vascular action. The current study determined the effect of GLP-1R agonist exenatide on postprandial EF in type 2 diabetes and the mechanisms underlying GLP-1R agonist-mediated vasodilation. Two crossover studies were conducted: 36 participants with type 2 diabetes received subcutaneous exenatide or placebo for 11 days and EF, and glucose and lipid responses to breakfast and lunch were determined; and 32 participants with impaired glucose tolerance (IGT) or diet-controlled type 2 diabetes had EF measured before and after intravenous exenatide, with or without the GLP-1R antagonist exendin-9. Mechanisms of GLP-1R agonist action were studied ex vivo on human subcutaneous adipose tissue arterioles and endothelial cells. Subcutaneous exenatide increased postprandial EF independent of reductions in plasma glucose and triglycerides. Intravenous exenatide increased fasting EF, and exendin-9 abolished this effect. Exenatide elicited eNOS activation and NO production in endothelial cells, and induced dose-dependent vasorelaxation and reduced high-glucose or lipid-induced endothelial dysfunction in arterioles ex vivo. These effects were reduced with AMPK inhibition. In conclusion, exenatide augmented postprandial EF in subjects with diabetes and prevented high-glucose and lipid-induced endothelial dysfunction in human arterioles. These effects were largely direct, via GLP-1R and AMPK activation.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures



Comment in
-
GLP-1R Agonists and Endothelial Dysfunction: More Than Just Glucose Lowering?Diabetes. 2015 Jul;64(7):2319-21. doi: 10.2337/db15-0366. Diabetes. 2015. PMID: 26106189 No abstract available.
References
-
- Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–658 - PubMed
-
- Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 2002;105:1567–1572 - PubMed
-
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 2007;115:2390–2397 - PubMed
-
- McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1992;35:771–776 - PubMed
-
- Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 1996;27:567–574 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

