Hypoxia induces an undifferentiated phenotype of oral keratinocytes in vitro
- PMID: 25720390
- PMCID: PMC4410785
- DOI: 10.1159/000371342
Hypoxia induces an undifferentiated phenotype of oral keratinocytes in vitro
Abstract
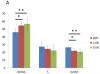
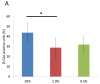
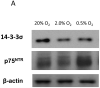
The aim of this study was to determine the effects of hypoxia on the proliferating potential and phenotype of primary human oral keratinocytes cultured at ambient oxygen tension (20%) or at different levels of hypoxia (2 and 0.5% O2). The effects of oxygen tensions on cellular metabolic activity, cell proliferation, clonogenicity and proliferation heterogeneity were measured. Cell cycle profiles were analyzed by a fluorescent-activated cell sorter, and p21(WAF1/CIP1) expression in the G0/G1 phase was also concomitantly quantitated. The expression levels of cell cycle regulatory proteins were examined by immunoblotting, and the cellular senescence was assessed by senescence-associated β-galactosidase staining. Basal and suprabasal keratinocyte phenotypes were determined by the expression levels of 14-3-3σ, p75(NTR) and α6 integrin. Despite having a lower metabolism, the proliferation rate and clonogenic potential were remarkably enhanced in hypoxic cells. The significantly higher percentage of cells in the G0/G1 phase under hypoxia and the expression patterns of cell cycle regulatory proteins in hypoxic cells were indicative of a state of cell cycle arrest in hypoxia. Furthermore, a decrease in the expression of p21(WAF1/CIP1) and p16(INK4A) and fewer β-galactosidase-positive cells suggested a quiescent phenotype rather than a senescent one in hypoxic cells. Compared with normoxic cells, the differential expression patterns of keratinocyte phenotypic markers suggest that hypoxic cells that generate minimal reactive oxygen species, suppress the mammalian target of rapamycin activity and express hypoxia-inducible factor-1α favor a basal cell phenotype. Thus, regardless of the predisposition to the state of cell cycle arrest, hypoxic conditions can maintain oral keratinocytes in vitro in an undifferentiated and quiescent state.
© 2015 S. Karger AG, Basel.
Figures















Similar articles
-
HIF-1alpha controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1).Biochim Biophys Acta. 2008 Feb;1783(2):323-33. doi: 10.1016/j.bbamcr.2007.11.017. Epub 2007 Dec 8. Biochim Biophys Acta. 2008. PMID: 18166158
-
Hypoxia inhibits cellular senescence to restore the therapeutic potential of old human endothelial progenitor cells via the hypoxia-inducible factor-1α-TWIST-p21 axis.Arterioscler Thromb Vasc Biol. 2013 Oct;33(10):2407-14. doi: 10.1161/ATVBAHA.113.301931. Epub 2013 Aug 8. Arterioscler Thromb Vasc Biol. 2013. PMID: 23928864
-
Knockdown of hypoxia-inducible factor-1 alpha reduces proliferation, induces apoptosis and attenuates the aggressive phenotype of retinoblastoma WERI-Rb-1 cells under hypoxic conditions.Ann Clin Lab Sci. 2014 Spring;44(2):134-44. Ann Clin Lab Sci. 2014. PMID: 24795051
-
[Impact of RNA interference targeting hypoxia-inducible factor-1alpha on chemosensitivity in esophageal squamous cell carcinoma cells under hypoxia].Zhonghua Yi Xue Za Zhi. 2007 Oct 9;87(37):2640-4. Zhonghua Yi Xue Za Zhi. 2007. PMID: 18162154 Chinese.
-
Modeling Preclinical Cancer Studies under Physioxia to Enhance Clinical Translation.Cancer Res. 2022 Dec 2;82(23):4313-4321. doi: 10.1158/0008-5472.CAN-22-2311. Cancer Res. 2022. PMID: 36169928 Free PMC article. Review.
Cited by
-
Noninvasive measurement of cell/colony motion using image analysis methods to evaluate the proliferative capacity of oral keratinocytes as a tool for quality control in regenerative medicine.J Tissue Eng. 2019 Oct 15;10:2041731419881528. doi: 10.1177/2041731419881528. eCollection 2019 Jan-Dec. J Tissue Eng. 2019. PMID: 31662840 Free PMC article.
-
Improvement of Antioxidant Defences in Keratinocytes Grown in Physioxia: Comparison of 2D and 3D Models.Oxid Med Cell Longev. 2023 Jun 17;2023:6829931. doi: 10.1155/2023/6829931. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 37360501 Free PMC article.
-
Metabolomic Alteration of Oral Keratinocytes and Fibroblasts in Hypoxia.J Clin Med. 2021 Mar 10;10(6):1156. doi: 10.3390/jcm10061156. J Clin Med. 2021. PMID: 33801898 Free PMC article.
References
-
- Bath C, Yang S, Muttuvelu D, Fink T, Emmersen J, Vorum H, Hjortdal J, Zachar V. Hypoxia is a key regulator of limbal epithelial stem cell growth and differentiation. Stem Cell Res. 2013;10:349–360. - PubMed
-
- Baris OR, Klose A, Kloepper JE, Weiland D, Neuhaus JF, Schauen M, et al. The mitochondrial electron transport chain is dispensable for proliferation and differentiation of epidermal progenitor cells. Stem Cells. 2011;29:1459–1468. - PubMed
-
- Buravkova LB, Rylova YV, Andreeva ER, Kulikov AV, Pogodina MV, Zhivotovsky B, et al. Low ATP level is sufficient to maintain the uncommitted state of multipotent mesenchymal stem cells. Biochim Biophys Acta. 2013;1830:4418–4425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

