Liquid biopsies in patients with diffuse glioma
- PMID: 25720744
- PMCID: PMC4436687
- DOI: 10.1007/s00401-015-1399-y
Liquid biopsies in patients with diffuse glioma
Abstract
Diffuse gliomas are the most common malignant primary tumors of the central nervous system. Like other neoplasms, these gliomas release molecular information into the circulation. Tumor-derived biomarkers include proteins, nucleic acids, and tumor-derived extracellular vesicles that accumulate in plasma, serum, blood platelets, urine and/or cerebrospinal fluid. Recently, also circulating tumor cells have been identified in the blood of glioma patients. Circulating molecules, vesicles, platelets, and cells may be useful as easily accessible diagnostic, prognostic and/or predictive biomarkers to guide patient management. Thereby, this approach may help to circumvent problems related to tumor heterogeneity and sampling error at the time of diagnosis. Also, liquid biopsies may allow for serial monitoring of treatment responses and of changes in the molecular characteristics of gliomas over time. In this review, we summarize the literature on blood-based biomarkers and their potential value for improving the management of patients with a diffuse glioma. Incorporation of the study of circulating molecular biomarkers in clinical trials is essential for further assessment of the potential of liquid biopsies in this context.
Figures
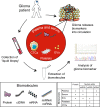
Similar articles
-
The emerging clinical potential of circulating extracellular vesicles for non-invasive glioma diagnosis and disease monitoring.Brain Tumor Pathol. 2019 Apr;36(2):29-39. doi: 10.1007/s10014-019-00335-0. Epub 2019 Mar 11. Brain Tumor Pathol. 2019. PMID: 30859343 Review.
-
Blood-based biomarkers for the diagnosis and monitoring of gliomas.Neuro Oncol. 2018 Aug 2;20(9):1155-1161. doi: 10.1093/neuonc/noy074. Neuro Oncol. 2018. PMID: 29746665 Free PMC article. Review.
-
Biomarkers in Cerebrospinal Fluid for the Diagnosis and Monitoring of Gliomas.Biomolecules. 2024 Jul 5;14(7):801. doi: 10.3390/biom14070801. Biomolecules. 2024. PMID: 39062515 Free PMC article. Review.
-
Cerebrospinal Fluid Liquid Biopsies in the Evaluation of Adult Gliomas.Curr Oncol Rep. 2024 Apr;26(4):377-390. doi: 10.1007/s11912-024-01517-6. Epub 2024 Mar 15. Curr Oncol Rep. 2024. PMID: 38488990 Review.
-
Clinically Relevant and Minimally Invasive Tumor Surveillance of Pediatric Diffuse Midline Gliomas Using Patient-Derived Liquid Biopsy.Clin Cancer Res. 2018 Dec 1;24(23):5850-5859. doi: 10.1158/1078-0432.CCR-18-1345. Epub 2018 Oct 15. Clin Cancer Res. 2018. PMID: 30322880 Free PMC article.
Cited by
-
Identification of Astrocytoma Blood Serum Protein Profile.Cells. 2019 Dec 19;9(1):16. doi: 10.3390/cells9010016. Cells. 2019. PMID: 31861636 Free PMC article.
-
MMP-9 as Prognostic Marker for Brain Tumours: A Comparative Study on Serum-Derived Small Extracellular Vesicles.Cancers (Basel). 2023 Jan 24;15(3):712. doi: 10.3390/cancers15030712. Cancers (Basel). 2023. PMID: 36765669 Free PMC article.
-
Glial biomarkers in human central nervous system disease.Glia. 2016 Oct;64(10):1755-71. doi: 10.1002/glia.22998. Epub 2016 May 26. Glia. 2016. PMID: 27228454 Free PMC article. Review.
-
New Frontiers in Diagnosis and Therapy of Circulating Tumor Markers in Cerebrospinal Fluid In Vitro and In Vivo.Cells. 2019 Oct 2;8(10):1195. doi: 10.3390/cells8101195. Cells. 2019. PMID: 31581745 Free PMC article. Review.
-
Extracellular Vesicles as a Novel Liquid Biopsy-Based Diagnosis for the Central Nervous System, Head and Neck, Lung, and Gastrointestinal Cancers: Current and Future Perspectives.Cancers (Basel). 2021 Jun 3;13(11):2792. doi: 10.3390/cancers13112792. Cancers (Basel). 2021. PMID: 34205183 Free PMC article. Review.
References
-
- Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S, Kalinina J, Hua W, Kesari S, Mao Y, Breakefield XO, Hochberg FH, Van Meir EG, Carter BS, Chen CC. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One. 2013;8:e78115. - PMC - PubMed
-
- Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–118. - PubMed
-
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

