IL10 and PD-1 Cooperate to Limit the Activity of Tumor-Specific CD8+ T Cells
- PMID: 25720800
- PMCID: PMC4401638
- DOI: 10.1158/0008-5472.CAN-14-3016
IL10 and PD-1 Cooperate to Limit the Activity of Tumor-Specific CD8+ T Cells
Abstract
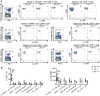
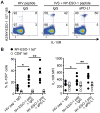
Immune checkpoint inhibitors show great promise as therapy for advanced melanoma, heightening the need to determine the most effective use of these agents. Here, we report that programmed death-1(high) (PD-1(high)) tumor antigen (TA)-specific CD8(+) T cells present at periphery and at tumor sites in patients with advanced melanoma upregulate IL10 receptor (IL10R) expression. Multiple subsets of peripheral blood mononucleocytes from melanoma patients produce IL10, which acts directly on IL10R(+) TA-specific CD8(+) T cells to limit their proliferation and survival. PD-1 blockade augments expression of IL10R by TA-specific CD8(+) T cells, thereby increasing their sensitivity to the immunosuppressive effects of endogenous IL10. Conversely, IL10 blockade strengthened the effects of PD-1 blockade in expanding TA-specific CD8(+) T cells and reinforcing their function. Collectively, our findings offer a rationale to block both IL10 and PD-1 to strengthen the counteraction of T-cell immunosuppression and to enhance the activity of TA-specific CD8(+) T cell in advanced melanoma patients.
©2015 American Association for Cancer Research.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures




References
-
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annual review of immunology. 2006;24:175–208. - PubMed
-
- Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–9. - PubMed
-
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

