The multi-targeted tyrosine kinase inhibitor vandetanib plays a bifunctional role in non-small cell lung cancer cells
- PMID: 25720956
- PMCID: PMC4342569
- DOI: 10.1038/srep08629
The multi-targeted tyrosine kinase inhibitor vandetanib plays a bifunctional role in non-small cell lung cancer cells
Abstract
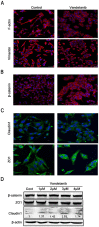
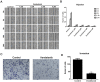
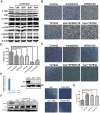
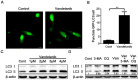
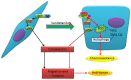
Vandetanib, a multikinase inhibitor, is a target of drug treatments for non-small cell lung cancer (NSCLC). However, phase II and III clinical trials have not conclusively demonstrated the curative effects of vandetanib for NSCLC, and the reasons for this are unknown. In the present study, we use the NSCLC cell line Calu-6 as a model to determine the cellular and biological effects of vandetanib. Our results demonstrate that vandetanib impairs Calu-6 cell migration and invasion. We find that vandetanib can directly inhibit RET activity, which influences the Rho-JNK pathway. Overexpression of a constitutively active Rho GTPase antagonizes the inhibitory effects of vandetanib on Calu-6 cells invasion and JNK pathway activation. In addition, vandetanib induces autophagy by increasing the level of reactive oxygen species (ROS) in Calu-6 cells, and blockade of autophagy or ROS effectively enhances the cell death effect of vandetanib. In this study, we find vandetanib is of a double effect in some NSCLC cells, presenting new possibilities for the pharmacological treatment of NSCLC and introducing a novel role for vandetanib in treatment options.
Figures


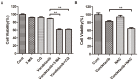
References
-
- da Cunha Santos G., Shepherd F. A. & Tsao M. S. EGFR mutations and lung cancer. Annu Rev Pathol 6, 49–69 (2011). - PubMed
-
- Mok T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361, 947–57 (2009). - PubMed
-
- Maemondo M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362, 2380–8 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

