Emerging roles for RNA degradation in viral replication and antiviral defense
- PMID: 25721579
- PMCID: PMC4424162
- DOI: 10.1016/j.virol.2015.02.007
Emerging roles for RNA degradation in viral replication and antiviral defense
Abstract
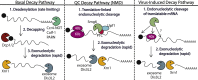
Viral replication significantly alters the gene expression landscape of infected cells. Many of these changes are driven by viral manipulation of host transcription or translation machinery. Several mammalian viruses encode factors that broadly dampen gene expression by directly targeting messenger RNA (mRNA). Here, we highlight how these factors promote mRNA degradation to globally regulate both host and viral gene expression. Although these viral factors are not homologous and use distinct mechanisms to target mRNA, many of them display striking parallels in their strategies for executing RNA degradation and invoke key features of cellular RNA quality control pathways. In some cases, there is a lack of selectivity for degradation of host versus viral mRNA, indicating that the purposes of virus-induced mRNA degradation extend beyond redirecting cellular resources towards viral gene expression. In addition, several antiviral pathways use RNA degradation as a viral restriction mechanism, and we will summarize new findings related to how these host-encoded ribonucleases target and destroy viral RNA.
Keywords: Endonuclease; Host shutoff; MRNA degradation; Xrn1.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

