Factors Associated With Plasma IL-6 Levels During HIV Infection
- PMID: 25722296
- PMCID: PMC4598808
- DOI: 10.1093/infdis/jiv123
Factors Associated With Plasma IL-6 Levels During HIV Infection
Abstract
Background: Elevated interleukin 6 (IL-6) levels have been linked to cardiovascular disease, cancer and death. Persons with human immunodeficiency virus (HIV) infection receiving treatment have higher IL-6 levels, but few data are available on factors associated with circulating IL-6.
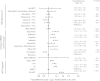
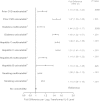
Methods: Participants in 3 trials with IL-6 measured at baseline were included (N = 9864). Factors associated with IL-6 were identified by linear regression. Demographic and HIV variables (nadir/entry CD4(+) cell count, HIV RNA level, antiretroviral therapy regimen) were investigated in all 3 trials. In the SMART (Strategies for Management of Anti-Retroviral Therapy) trial, CD4/CD8 ratio, smoking, comorbid conditions, serum lipids, renal function (estimated glomerular filtration rate [eGFR]), and educational level were assessed.
Results: Demographics associated with higher IL-6 levels were older age and lower education, whereas black race was associated with lower IL-6. Higher HIV RNA levels were associated with higher IL-6 levels, and higher nadir CD4(+) cell counts with lower IL-6 levels. Compared with efavirenz, protease inhibitors were associated with higher and nevirapine with lower IL-6 levels. Smoking and all comorbid conditions were related to higher IL-6. IL-6 levels increased with decreasing eGFR and decreasing serum lipids.
Conclusions: Higher levels of IL-6 were associated with older age, nonblack race, higher body mass index, lower serum lipid levels, HIV replication, low nadir CD4(+) cell count, protease inhibitor use, comorbid conditions, and decreased eGFR. Multiple factors affect inflammation in HIV and should be considered in studies of IL-6 as a biomarker of clinical outcomes.
Keywords: HIV; IL-6; cancer; cardiovascular disease; inflammation.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res 2014; 2:288–94. - PubMed
-
- Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 2003; 24:25–9. - PubMed
-
- Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med 2000; 6:583–8. - PubMed
-
- Nakajima K, Martínez-Maza O, Hirano T, et al. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol 1989; 142:531–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

