A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)†
- PMID: 25722381
- PMCID: PMC4855243
- DOI: 10.1093/annonc/mdv072
A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)†
Abstract
Background: KRAS mutations are detected in 25% of non-small-cell lung cancer (NSCLC) and no targeted therapies are approved for this subset population. Trametinib, a selective allosteric inhibitor of MEK1/MEK2, demonstrated preclinical and clinical activity in KRAS-mutant NSCLC. We report a phase II trial comparing trametinib with docetaxel in patients with advanced KRAS-mutant NSCLC.
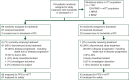
Patients and methods: Eligible patients with histologically confirmed KRAS-mutant NSCLC previously treated with one prior platinum-based chemotherapy were randomly assigned in a ratio of 2 : 1 to trametinib (2 mg orally once daily) or docetaxel (75 mg/m(2) i.v. every 3 weeks). Crossover to the other arm after disease progression was allowed. Primary end point was progression-free survival (PFS). The study was prematurely terminated after the interim analysis of 92 PFS events, which showed the comparison of trametinib versus docetaxel for PFS crossed the futility boundary.
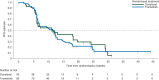
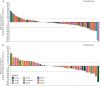
Results: One hundred and twenty-nine patients with KRAS-mutant NSCLC were randomized; of which, 86 patients received trametinib and 43 received docetaxel. Median PFS was 12 weeks in the trametinib arm and 11 weeks in the docetaxel arm (hazard ratio [HR] 1.14; 95% CI 0.75-1.75; P = 0.5197). Median overall survival, while the data are immature, was 8 months in the trametinib arm and was not reached in the docetaxel arm (HR 0.97; 95% CI 0.52-1.83; P = 0.934). There were 10 (12%) partial responses (PRs) in the trametinib arm and 5 (12%) PRs in the docetaxel arm (P = 1.0000). The most frequent adverse events (AEs) in ≥20% of trametinib patients were rash, diarrhea, nausea, vomiting, and fatigue. The most frequent grade 3 treatment-related AEs in the trametinib arm were hypertension, rash, diarrhea, and asthenia.
Conclusion: Trametinib showed similar PFS and a response rate as docetaxel in patients with previously treated KRAS-mutant-positive NSCLC.
Clinicaltrialsgov registration number: NCT01362296.
Keywords: KRAS; MEK inhibitor; NSCLC; docetaxel; progression-free survival; trametinib.
© The Author 2015. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009; 6: 201–205. - PubMed
-
- Sanders HR, Albitar M. Somatic mutations of signaling genes in non-small-cell lung cancer. Cancer Genet Cytogenet 2010; 203: 7–15. - PubMed
-
- Okudela K, Woo T, Kitamura H. KRAS gene mutations in lung cancer: particulars established and issues unresolved. Pathol Int 2010; 60: 651–660. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

