Electrocardiographic imaging of heart rhythm disorders: from bench to bedside
- PMID: 25722753
- PMCID: PMC4337422
- DOI: 10.1016/j.ccep.2014.11.013
Electrocardiographic imaging of heart rhythm disorders: from bench to bedside
Abstract
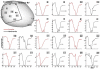
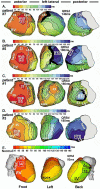
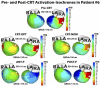
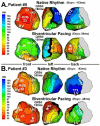
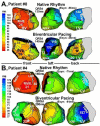
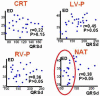
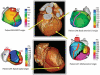
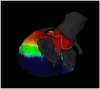
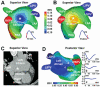
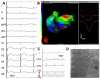
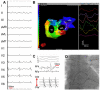
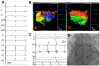
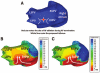
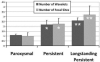
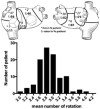
Noninvasive electrocardiographic imaging (ECGI; also called ECG mapping) can reconstruct potentials, electrograms, activation sequences, and repolarization patterns on the epicardial surface of the heart with high resolution. ECGI can possibly be used to quantify synchrony, identify potential responders/nonresponders to cardiac resynchronization therapy, and guide electrode placement for effective resynchronization therapy. This article provides a brief description of the ECGI procedure and selected previously published examples of its application in important clinical conditions, including heart failure, cardiac resynchronization therapy, atrial arrhythmias, and ventricular tachycardia.
Figures





References
-
- Plonsey R, Barr RC. Bioelectricity—a quantitative approach. 3rd Springer; New York: 2007.
-
- Barr RC, Ramsey M, III, Spach MS. Relating epicardial to body surface potential distributions by means of transfer coefficients based on geometry measurements. IEEE Trans Biomed Eng. 1977;24:1–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

