The future of high-grade glioma: Where we are and where are we going
- PMID: 25722939
- PMCID: PMC4338495
- DOI: 10.4103/2152-7806.151331
The future of high-grade glioma: Where we are and where are we going
Erratum in
-
Erratum: The future of high-grade glioma: Where we are and where are we going: Erratum.Surg Neurol Int. 2015 Mar 5;6:37. doi: 10.4103/2152-7806.153100. eCollection 2015. Surg Neurol Int. 2015. PMID: 25789199 Free PMC article.
Abstract
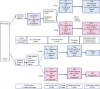
High-grade glioma (HGG) are optimally treated with maximum safe surgery, followed by radiotherapy (RT) and/or systemic chemotherapy (CT). Recently, the treatment of newly diagnosed anaplastic glioma (AG) has changed, particularly in patients with 1p19q codeleted tumors. Results of trials currenlty ongoing are likely to determine the best standard of care for patients with noncodeleted AG tumors. Trials in AG illustrate the importance of molecular characterization, which are germane to both prognosis and treatment. In contrast, efforts to improve the current standard of care of newly diagnosed glioblastoma (GB) with, for example, the addition of bevacizumab (BEV), have been largely disappointing and furthermore molecular characterization has not changed therapy except in elderly patients. Novel approaches, such as vaccine-based immunotherapy, for newly diagnosed GB are currently being pursued in multiple clinical trials. Recurrent disease, an event inevitable in nearly all patients with HGG, continues to be a challenge. Both recurrent GB and AG are managed in similar manner and when feasible re-resection is often suggested notwithstanding limited data to suggest benefit from repeat surgery. Occassional patients may be candidates for re-irradiation but again there is a paucity of data to commend this therapy and only a minority of selected patients are eligible for this approach. Consequently systemic therapy continues to be the most often utilized treatment in recurrent HGG. Choice of therapy, however, varies and revolves around re-challenge with temozolomide (TMZ), use of a nitrosourea (most often lomustine; CCNU) or BEV, the most frequently used angiogenic inhibitor. Nevertheless, no clear standard recommendation regarding the prefered agent or combination of agents is avaliable. Prognosis after progression of a HGG remains poor, with an unmet need to improve therapy.
Keywords: Anaplastic glioma; anti-angiogenic agents; bevacizumab; chemotherapy; glioblastoma; high grade glioma; immunotherapy; nitrosourea; targeted therapy; temozolomide.
Figures




References
-
- Abstracts from the World Federation of Neuro-Oncology Second Quadrennial Meeting and the Sixth Meeting of the European Association for Neuro-Oncology. Neuro Oncol. 2005;7:279–402.
-
- Affronti ML, Heery CR, Herndon JE, 2nd, Rich JN, Reardon DA, Desjardins A, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115:3501–11. - PubMed
-
- Ali SA, McHayleh WM, Ahmad A, Sehgal R, Braffet M, Rahman M, et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: A series of 13 cases. J Neurosurg. 2008;109:268–72. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

