Antiretroviral Treatment Is Associated With Iron Deficiency in HIV-Infected Malawian Women That Is Mitigated With Supplementation, but Is Not Associated With Infant Iron Deficiency During 24 Weeks of Exclusive Breastfeeding
- PMID: 25723140
- PMCID: PMC4506710
- DOI: 10.1097/QAI.0000000000000588
Antiretroviral Treatment Is Associated With Iron Deficiency in HIV-Infected Malawian Women That Is Mitigated With Supplementation, but Is Not Associated With Infant Iron Deficiency During 24 Weeks of Exclusive Breastfeeding
Erratum in
- J Acquir Immune Defic Syndr. 2015 Aug 15;69(5):e184
Abstract
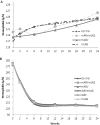
Objective: In resource-limited settings without safe alternatives to breastfeeding, the WHO recommends exclusive breastfeeding and antiretroviral (ARV) prophylaxis. Given the high prevalence of anemia among HIV-infected women, mothers and their infants (through fetal iron accretion) may be at risk of iron deficiency. We assessed the effects of maternal micronutrient-fortified lipid-based nutrient supplements (LNS) and maternal ARV treatment or infant ARV prophylaxis on maternal and infant iron status during exclusive breastfeeding from birth to 24 weeks.
Methods: The Breastfeeding, Antiretrovirals, and Nutrition study was a randomized controlled trial conducted in Lilongwe, Malawi, from 2004 to 2010. HIV-infected mothers (CD4 >200 cells/μL) and their infants were randomly assigned to 28-week interventions: maternal LNS/maternal ARV (n = 424), maternal LNS/infant ARV (n = 426), maternal LNS (n = 334), maternal ARV (n = 425), infant ARV (n = 426), or control (n = 334). Longitudinal models tested intervention effects on hemoglobin (Hb). In a subsample (n = 537) with multiple iron indicators, intervention effects on Hb, transferrin receptors (TfR), and ferritin were tested with linear and Poisson regression.
Results: In longitudinal models, LNS effects on maternal and infant Hb were minimal. In subsample mothers, maternal ARVs were associated with tissue iron depletion (TfR >8.3 mg/L) (risk ratio: 3.1, P < 0.01), but not in ARV-treated mothers receiving LNS (P = 0.17). LNS without ARVs was not associated with iron deficiency or anemia (P > 0.1). In subsample infants, interventions were not associated with impaired iron status (all P > 0.1).
Conclusions: Maternal ARV treatment with protease inhibitors is associated with maternal tissue iron depletion; but LNS mitigates adverse effects. ARVs do not seem to influence infant iron status; however, extended use needs to be evaluated.
Trial registration: ClinicalTrials.gov NCT00164736.
Conflict of interest statement
Author disclosure: C.M. van der Horst received grant support from Abbott Laboratories and GlaxoSmithKline. No other conflicts of interest were reported.
The University of North Carolina received grant support from Abbott Laboratories and GlaxoSmithKline. All other authors declare that they have no conflicts of interest.
Figures
References
-
- World Health Organization. Guidelines on HIV and Infant Feeding: Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva, Switzerland: WHO Press; 2010. - PubMed
-
- Papathakis PC, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. Am J Clin Nutr. 2007;85(1):182–192. - PubMed
-
- Friis H, Gomo E, Koestel P, et al. HIV and other predictors of serum folate, serum ferritin, and hemoglobin in pregnancy: a cross-sectional study in Zimbabwe. Am J Clin Nutr. 2001;73(6):1066–1073. - PubMed
-
- O’Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. 2005 Oct 1;40(2):219–225. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- SIP 22-09 U48-DP001944-01/DP/NCCDPHP CDC HHS/United States
- D43 TW001039/TW/FIC NIH HHS/United States
- SIP 26-04 U48-DP000059-01/DP/NCCDPHP CDC HHS/United States
- U48DP001944/ACL/ACL HHS/United States
- R24 TW007988/TW/FIC NIH HHS/United States
- R24 HD050924/HD/NICHD NIH HHS/United States
- P2C HD050924/HD/NICHD NIH HHS/United States
- U48 DP000059/DP/NCCDPHP CDC HHS/United States
- 2-D43TW01039-06/TW/FIC NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- R03 HD057775/HD/NICHD NIH HHS/United States
- SIP 13-01 U48-CCU409660-09/PHS HHS/United States
- R03 HD057637/HD/NICHD NIH HHS/United States
- P30-AI50410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

