Efficacy and safety of oral palonosetron compared with IV palonosetron administered with dexamethasone for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with solid tumors receiving cisplatin-based highly emetogenic chemotherapy (HEC)
- PMID: 25724407
- PMCID: PMC4552772
- DOI: 10.1007/s00520-015-2657-1
Efficacy and safety of oral palonosetron compared with IV palonosetron administered with dexamethasone for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with solid tumors receiving cisplatin-based highly emetogenic chemotherapy (HEC)
Abstract
Purpose: This study aims to compare the efficacy and safety of oral palonosetron with intravenous (IV) palonosetron for the prevention of cisplatin-related chemotherapy-induced nausea and vomiting (CINV).
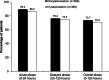
Methods: A multinational, randomized, double-blind study enrolling adult chemotherapy-naive patients with malignant solid tumors scheduled to receive cisplatin-based highly emetogenic chemotherapy (HEC). Patients received oral palonosetron (0.50 mg) or IV palonosetron (0.25 mg), each with oral dexamethasone. The primary objective was to demonstrate non-inferiority in terms of patients with a complete response (CR, no emesis/no rescue medication) within the acute phase (0-24 h after chemotherapy administration).
Results: Of the 743 patients randomized, 739 received study medications and 738 were included in the full analysis set. The CR rate in the acute phase was high for both groups (oral 89.4 %; IV 86.2 %). As this difference in proportions (stratum-adjusted Cochran-Mantel-Haenszel method) was 3.21 % (99 % confidence interval (CI) -2.74 to 9.17 %), non-inferiority was demonstrated (since the lower limit of the 99 % CI was closer to zero than the predefined margin of 15 %). Treatment-emergent adverse events (TEAEs) related to the study drug were rare (oral 3.2 %; IV 6.5 %). No TEAEs related to study drug leading to discontinuation were reported.
Conclusion: Non-inferiority of oral versus IV palonosetron was demonstrated. The CR rate in the acute phase was >86 % in both patient groups. The safety profiles were comparable.
Figures
References
-
- Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D, Group EMGW Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. - DOI - PubMed
-
- Boccia R, Grunberg S, Franco-Gonzales E, Rubenstein E, Voisin D. Efficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: a phase 3 trial. Support Care Cancer. 2013;21(5):1453–1460. doi: 10.1007/s00520-012-1691-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials