Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice
- PMID: 25724490
- PMCID: PMC4551118
- DOI: 10.1152/ajpheart.00465.2014
Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice
Abstract
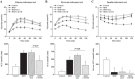
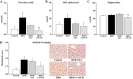
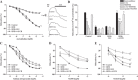
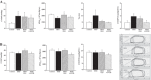
This study addressed the hypothesis that inhibiting the soluble epoxide hydrolase (sEH)-mediated degradation of epoxy-fatty acids, notably epoxyeicosatrienoic acids, has an additional impact against cardiovascular damage in insulin resistance, beyond its previously demonstrated beneficial effect on glucose homeostasis. The cardiovascular and metabolic effects of the sEH inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB; 10 mg/l in drinking water) were compared with those of the sulfonylurea glibenclamide (80 mg/l), both administered for 8 wk in FVB mice subjected to a high-fat diet (HFD; 60% fat) for 16 wk. Mice on control chow diet (10% fat) and nontreated HFD mice served as controls. Glibenclamide and t-AUCB similarly prevented the increased fasting glycemia in HFD mice, but only t-AUCB improved glucose tolerance and decreased gluconeogenesis, without modifying weight gain. Moreover, t-AUCB reduced adipose tissue inflammation, plasma free fatty acids, and LDL cholesterol and prevented hepatic steatosis. Furthermore, only the sEH inhibitor improved endothelium-dependent relaxations to acetylcholine, assessed by myography in isolated coronary arteries. This improvement was related to a restoration of epoxyeicosatrienoic acid and nitric oxide pathways, as shown by the increased inhibitory effects of the nitric oxide synthase and cytochrome P-450 epoxygenase inhibitors l-NA and MSPPOH on these relaxations. Moreover, t-AUCB decreased cardiac hypertrophy, fibrosis, and inflammation and improved diastolic function, as demonstrated by the increased E/A ratio (echocardiography) and decreased slope of the end-diastolic pressure-volume relation (invasive hemodynamics). These results demonstrate that sEH inhibition improves coronary endothelial function and prevents cardiac remodeling and diastolic dysfunction in obese insulin-resistant mice.
Keywords: cardiac function; endothelium; insulin resistance; soluble epoxide hydrolase.
Copyright © 2015 the American Physiological Society.
Figures




References
-
- Banquet S, Gomez E, Nicol L, Edwards-Lévy F, Henry JP, Cao R, Schapman D, Dautreaux B, Lallemand F, Bauer F, Cao Y, Thuillez C, Mulder P, Richard V, Brakenhielm E. Arteriogenic therapy by intramyocardial sustained delivery of a novel growth factor combination prevents chronic heart failure. Circulation 124: 1059–1069, 2011. - PubMed
-
- Bellien J, Iacob M, Remy-Jouet I, Lucas D, Monteil C, Gutierrez L, Vendeville C, Dreano Y, Mercier A, Thuillez C, Joannides R. Epoxyeicosatrienoic acids contribute with altered NO and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation 125: 1266–1275, 2012. - PubMed
-
- Bellien J, Joannides R, Richard V, Thuillez C. Modulation of cytochrome-derived epoxyeicosatrienoic acids pathway: a promising pharmacological approach to prevent endothelial dysfunction in cardiovascular diseases? Pharmacol Ther 131: 1–17, 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

