Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib
- PMID: 25724526
- PMCID: PMC4470734
- DOI: 10.1158/1078-0432.CCR-14-3009
Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib
Abstract
Purpose: Anaplastic lymphoma kinase (ALK) rearrangements are important therapeutic targets in non-small cell lung cancer (NSCLC) that confer sensitivity to the ALK inhibitors crizotinib and ceritinib. To determine the outcome of sequential treatment with crizotinb and ceritinib, we retrospectively evaluated a cohort of ALK-positive patients treated with both agents.
Experimental design: We identified 73 ALK-positive NSCLC patients treated with crizotinib followed by ceritinib at four institutions. Medical records were reviewed to determine overall survival (OS) and progression-free survival (PFS) on crizotinib and ceritinib.
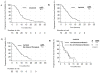
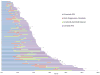
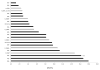
Results: Among 73 ALK-positive patients, the median PFS (mPFS) on crizotinib was 8.2 months [95% confidence interval (CI), 7.4-10.6]. The median interval from crizotinib discontinuation to initiation of ceritinib was 25 days (range, 1-694). The mPFS on ceritinib was 7.8 months (6.5-9.1). Among 53 patients with no interval therapies between crizotinib and ceritinib, the mPFS on ceritinib was similar at 7.8 months (5.4-9.8). The median combined PFS for sequential treatment with crizotinib and ceritinib was 17.4 months (15.5-19.4). Among 23 patients who underwent post-crizotinib/pre-ceritinib biopsies, there was no difference in PFS on ceritinib between patients with or without ALK resistance mutations (mPFS 5.8 vs. 6.5 months, respectively; P = 0.510). In the overall study population, median OS was 49.4 months (35.5-63.1).
Conclusions: Ceritinib has significant antitumor activity in ALK-positive NSCLC-even when crizotinib immediately precedes treatment with ceritinib (median combined PFS 17.0 months). Additional studies are necessary to further define the impact of specific ALK resistance mutations on duration of response to ceritinib.
©2015 American Association for Cancer Research.
Figures
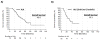
References
-
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

