Baseline Attitudes About Prostate Cancer Screening Moderate the Impact of Decision Aids on Screening Rates
- PMID: 25724634
- PMCID: PMC4959888
- DOI: 10.1007/s12160-015-9692-5
Baseline Attitudes About Prostate Cancer Screening Moderate the Impact of Decision Aids on Screening Rates
Abstract
Background: The impact of decision aids on prostate cancer screening outcomes has been inconsistent.
Purpose: We assessed whether pre-existing attitudes moderated the impact of decision aids on screening.
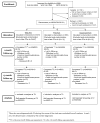
Methods: Men aged 45-70 (56.2% Caucasian, 39.9% African-American) were randomly assigned to a print decision aid (N = 630), a web decision aid (N = 631), or usual care (N = 632). Telephone interviews assessed pro/con screening attitudes and screening behaviors at baseline, 1-month and 13-months post-randomization.
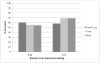
Results: Logistic regression analyses revealed significant arm by attitude interactions: Higher baseline cons scores predicted lower screening in the print (OR = 0.60 (95% CI: 0.40, 0.92)) and web (OR = 0.61 (95% CI: 0.40, 0.91)) arms but not in usual care (OR = 1.34 (95% CI: 0.90, 2.00)).
Conclusions: The decision aids amplified the impact of men's baseline attitudes about limitations of screening: Compared to the usual care arm, men in both decision aid arms were less likely to be screened when they perceived more limitations of screening.
Conflict of interest statement
Figures
References
-
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Accessed June 23, 2014]. Available at http://www.cancer.org/acs/groups/content/@research/documents/webcontent/....
-
- Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51. - PubMed
-
- Justman S. Uninformed consent: mass screening for prostate cancer. Bioethics. 2012;26:143–148. - PubMed
-
- Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical