Under-reporting and Poor Adherence to Monitoring Guidelines for Severe Cases of Isoniazid Hepatotoxicity
- PMID: 25724701
- PMCID: PMC4653068
- DOI: 10.1016/j.cgh.2015.02.024
Under-reporting and Poor Adherence to Monitoring Guidelines for Severe Cases of Isoniazid Hepatotoxicity
Abstract
Background & aims: Isoniazid is a leading cause of liver injury but it is not clear how many cases are reported or how many clinicians and patients adhere to American Thoracic Society (ATS) guidelines. We collected data on cases of isoniazid hepatotoxicity and assessed adherence to ATS guidelines and reports to the Centers for Disease Control's (CDC) isoniazid severe adverse events program.
Methods: We analyzed Drug-Induced Liver Injury Network (DILIN) cases considered definite, highly likely, or probable for isoniazid injury from 2004 through 2013. We assessed the delays in isoniazid discontinuance according to ATS criteria and hepatotoxicity severity by Severity Index Score. We checked reporting to the CDC by matching cases based on age, latency, indication, reporting period, and comorbidities.
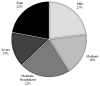
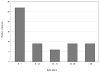
Results: Isoniazid was the second most commonly reported agent in the DILIN, with 69 cases; 60 of these met inclusion criteria. The median age of cases was 49 years (range, 4-68 y), 70% were female, 97% had latent tuberculosis, and 62% were hospitalized. Patients took a median of 9 days to stop taking isoniazid (range, 0-99 days). Thirty-three cases (55%) continued taking isoniazid for more than 7 days after the ATS criteria for stopping were met. Twenty-four cases (40%) continued isoniazid for more than 14 days after meeting criteria for stopping. A delay in stopping was associated with more severe injury (P < .05). Of 13 patients who died or underwent liver transplantation, 9 (70%) continued taking isoniazid for more than 7 days after meeting criteria for stopping. Only 1 of 25 cases of isoniazid hepatotoxicity eligible for reporting to the CDC was reported.
Conclusions: Poor adherence to ATS guidelines is common in cases of hepatotoxicity and is associated with more severe outcomes including hospitalization, death, and liver transplantation. Isoniazid continues to be a leading cause of DILI in the United States, and its hepatotoxicity is under-reported significantly.
Keywords: Adverse Reaction; Antibiotics; Drug-Induced Liver Injury; Hepatotoxicity; Tuberculosis.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Reducing Risk of Severe Liver Injury in Patients Treated With Isoniazid.Clin Gastroenterol Hepatol. 2015 Sep;13(9):1683-5. doi: 10.1016/j.cgh.2015.04.021. Epub 2015 Apr 27. Clin Gastroenterol Hepatol. 2015. PMID: 25929538 No abstract available.
References
-
- Garibaldi RA, Drusin RE, Ferebee SH, Gregg MB. Isoniazid-associated hepatitis. Report of an outbreak. Am Rev Respir Dis. 1972 Sep;106(3):357–365. - PubMed
-
- Runyon EH. Preventive Treatment in Tuberculosis: A Statement by the Committee on Therapy, American Thoracic Society. Am Rev Respir Dis. 1965 Feb;91:297–298. - PubMed
-
- Randolph H, Joseph S. Toxic hepatitis with jaundice occuring in a patient treated with isoniazid. J Am Med Assoc. 1953 May 2;152(1):38–40. - PubMed
-
- Gellis SN, Murphy RV. Hepatitis following isoniazid. Dis Chest. 1955 Oct;28(4):462–464. - PubMed
-
- [accessed Jun 6, 2013];LiverTox: Isoniazid. http://livertox.nlm.nih.gov/Isoniazid.htm.
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK065211/DK/NIDDK NIH HHS/United States
- 2U01-DK065176-06/DK/NIDDK NIH HHS/United States
- UL1 TR001105/TR/NCATS NIH HHS/United States
- 2U01-DK065201-06/DK/NIDDK NIH HHS/United States
- U01 DK083020/DK/NIDDK NIH HHS/United States
- U01 DK065238/DK/NIDDK NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- U01 DK065176/DK/NIDDK NIH HHS/United States
- U01 DK083023/DK/NIDDK NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- 5U01-DK065238-08/DK/NIDDK NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- 5U01DK065193-04/DK/NIDDK NIH HHS/United States
- U01 DK083027/DK/NIDDK NIH HHS/United States
- U01 DK065201/DK/NIDDK NIH HHS/United States
- 1U01-DK082992-01/DK/NIDDK NIH HHS/United States
- U01 DK065193/DK/NIDDK NIH HHS/United States
- 2U01-DK065184-06/DK/NIDDK NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- 2U01-DK065211-06/DK/NIDDK NIH HHS/United States
- 1U01-DK083020-01/DK/NIDDK NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- 1U01-DK083023-01/DK/NIDDK NIH HHS/United States
- UL1 RR024150/RR/NCRR NIH HHS/United States
- 1U01-DK083027-01/DK/NIDDK NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- U01 DK065184/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- U01 DK082992/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

