Antibody Drug Conjugates: Preclinical Considerations
- PMID: 25724883
- PMCID: PMC4406963
- DOI: 10.1208/s12248-015-9738-4
Antibody Drug Conjugates: Preclinical Considerations
Abstract
The development path for antibody drug conjugates (ADCs) is more complex and challenging than for unmodified antibodies. While many of the preclinical considerations for both unmodified and antibody drug conjugates are shared, special considerations must be taken into account when developing an ADC. Unlike unmodified antibodies, an ADC must preferentially bind to tumor cells, internalize, and traffic to the appropriate intracellular compartment to release the payload. Parameters that can impact the pharmacological properties of this class of therapeutics include the selection of the payload, the type of linker, and the methodology for payload drug conjugation. Despite a plethora of in vitro assays and in vivo models to screen and evaluate ADCs, the challenge remains to develop improved preclinical tools that will be more predictive of clinical outcome. This review will focus on preclinical considerations for clinically validated small molecule ADCs. In addition, the lessons learned from Mylotarg®, the first in class FDA-approved ADC, are highlighted.
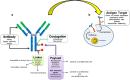
Figures
References
-
- Decarvalho S, Rand HJ, Lewis A. Coupling of cyclic chemotherapeutic compounds to immune gamma-globulins. Nature. 1964;202:255–8. - PubMed
-
- Mullard A. Maturing antibody-drug conjugate pipeline hits 30. Nat Rev Drug Discov. 2013;12(5):329–32. - PubMed
-
- Zheng B, Fuji RN, Elkins K, et al. In vivo effects of targeting CD79b with antibodies and antibody-drug conjugates. Mol Cancer Ther. 2009;8(10):2937–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

