Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies
- PMID: 25726085
- PMCID: PMC4401426
- DOI: 10.1016/S2213-2600(15)00036-3
Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies
Abstract
Background: Sputum culture conversion is often used as an early microbiological endpoint in phase 2 clinical trials of tuberculosis treatment on the basis of its assumed predictive value for end-of-treatment outcome, particularly in patients with drug-susceptible tuberculosis. We aimed to assess the validity of sputum culture conversion on solid media at varying timepoints, and the time to conversion, as prognostic markers for end-of-treatment outcome in patients with multidrug-resistant (MDR) tuberculosis.
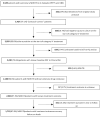
Methods: We analysed data from two large cohort studies of patients with MDR tuberculosis. We defined sputum culture conversion as two or more consecutive negative cultures from sputum samples obtained at least 30 days apart. To estimate the association of 2 month and 6 month conversion with successful treatment outcome, we calculated odds ratios (ORs) and 95% CIs with random-effects multivariable logistic regression. We calculated predictive values with bivariate random-effects generalised linear mixed modelling.
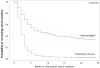
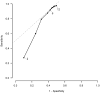
Findings: We assessed data for 1712 patients who had treatment success, treatment failure, or who died. Among patients with treatment success, median time to sputum culture conversion was significantly shorter than in those who had poor outcomes (2 months [IQR 1-3] vs 7 months [3 to ≥24]; log-rank p<0·0001). Furthermore, conversion status at 6 months (adjusted OR 14·07 [95% CI 10·05-19·71]) was significantly associated with treatment success compared with failure or death. Sputum culture conversion status at 2 months was significantly associated with treatment success only in patients who were HIV negative (adjusted OR 4·12 [95% CI 2·25-7·54]) or who had unknown HIV infection (3·59 [1·96-6·58]), but not in those who were HIV positive (0·38 [0·12-1·18]). Thus, the overall association of sputum culture conversion with a successful outcome was substantially greater at 6 months than at 2 months. 2 month conversion had low sensitivity (27·3% [95% confidence limit 16·6-41·4]) and high specificity (89·8% [82·3-94·4]) for prediction of treatment success. Conversely, 6 month sputum culture conversion status had high sensitivity (91·8% [85·9-95·4]), but moderate specificity (57·8% [42·5-71·6]). The maximum combined sensitivity and specificity for sputum culture conversion was reached between month 6 and month 10 of treatment.
Interpretation: Time to sputum culture conversion, conversion status at 6 months, and conversion status at 2 months in patients without known HIV infection can be considered as proxy markers of end-of-treatment outcome in patients with MDR tuberculosis, although the overall association with treatment success is substantially stronger for 6 month than for 2 month conversion status. Investigators should consider these results regarding the validity of sputum culture conversion at various timepoints as an early predictor of treatment efficacy when designing phase 2 studies before investing substantial resources in large, long-term, phase 3 trials of new treatments for MDR tuberculosis.
Funding: US Agency for International Development, US Centers for Disease Control and Prevention, Division of Intramural Research of the US National Institute of Allergy and Infectious Diseases, Korea Centers for Disease Control and Prevention.
Copyright © 2015 World Health Organization. Published by Elsevier Ltd/Inc/BV. All rights reserved. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
No conflict of interest reported for all contributors.
Figures

Comment in
-
Sputum culture conversion as a tuberculosis biomarker: a glass half empty or half full?Lancet Respir Med. 2015 Mar;3(3):174-5. doi: 10.1016/S2213-2600(15)00058-2. Epub 2015 Feb 26. Lancet Respir Med. 2015. PMID: 25726087 No abstract available.
References
-
- Food and Drug Administration (U.S.) [13 March 2014];FDA News Release. 2012 Dec 31;
-
- Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. The American review of respiratory disease. 1993 Apr;147(4):1062–1063. - PubMed
-
- Wallis RS, Doherty TM, Onyebujoh P, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis. 2009 Mar;9(3):162–172. - PubMed
-
- Lienhardt C, Davies G. Methodological issues in the design of clinical trials for the treatment of multidrug-resistant tuberculosis: challenges and opportunities. Int J Tuberc Lung Dis. 2010 May;14(5):528–537. - PubMed
-
- US Food and Drug Administration. Development of Drugs to Treat Multi-Drug Resistant Tuberculosis (MDR-TB). AIDAC Advisory Committee Meeting; June 3, 2009; [March 17, 2014]. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/A....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

