Hearing and music in dementia
- PMID: 25726296
- PMCID: PMC4809670
- DOI: 10.1016/B978-0-444-62630-1.00037-8
Hearing and music in dementia
Abstract
Music is a complex acoustic signal that relies on a number of different brain and cognitive processes to create the sensation of hearing. Changes in hearing function are generally not a major focus of concern for persons with a majority of neurodegenerative diseases associated with dementia, such as Alzheimer disease (AD). However, changes in the processing of sounds may be an early, and possibly preclinical, feature of AD and other neurodegenerative diseases. The aim of this chapter is to review the current state of knowledge concerning hearing and music perception in persons who have a dementia as a result of a neurodegenerative disease. The review focuses on both peripheral and central auditory processing in common neurodegenerative diseases, with a particular focus on the processing of music and other non-verbal sounds. The chapter also reviews music interventions used for persons with neurodegenerative diseases.
Keywords: Alzheimer's disease; Parkinson's disease; auditory evoked potentials; corticobasal degeneration; environmental sounds; frontotemporal dementia; melody; neurodegenerative disease; progressive supranuclear palsy; rhythm.
© 2015 Elsevier B.V. All rights reserved.
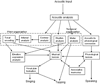
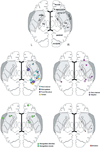
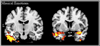
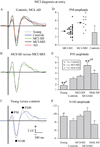
Figures


References
-
- Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. - PubMed
-
- Alzheimer's Association. 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. - PubMed
-
- Amaducci L, Grassi E, Boller F. Maurice Ravel and right-hemisphere musical creativity: influence of disease on his last musical works? European Journal of Neurology. 2002;9(1):75–82. - PubMed
-
- American Psychiatric Association. text revision. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders.
-
- Ames D, Burns A, O’Brien J. Dementia. Boca Raton, Fl: CRC Press; 2010.

