Biliverdin reductase isozymes in metabolism
- PMID: 25726384
- PMCID: PMC4380527
- DOI: 10.1016/j.tem.2015.02.001
Biliverdin reductase isozymes in metabolism
Abstract
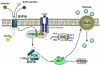
The biliverdin reductase (BVR) isozymes BVRA and BVRB are cell surface membrane receptors with pleiotropic functions. This review compares, for the first time, the structural and functional differences between the isozymes. They reduce biliverdin, a byproduct of heme catabolism, to bilirubin, display kinase activity, and BVRA, but not BVRB, can act as a transcription factor. The binding motifs present in the BVR isozymes allow a wide range of interactions with components of metabolically important signaling pathways such as the insulin receptor kinase cascades, protein kinases (PKs), and inflammatory mediators. In addition, serum bilirubin levels have been negatively associated with abdominal obesity and hypertriglyceridemia. We discuss the roles of the BVR isozymes in metabolism and their potential as therapeutic targets.
Keywords: BVRA; BVRB; bilirubin; biliverdin reductase; diabetes; heme oxygenase; obesity.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Jr., Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG. Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem cell research & therapy. 2013;4:28. - PMC - PubMed
-
- Li M, Peterson S, Husney D, Inaba M, Guo K, Terada E, Morita T, Patil K, Kappas A, Ikehara S, Abraham NG. Interdiction of the diabetic state in NOD mice by sustained induction of heme oxygenase: possible role of carbon monoxide and bilirubin. Antioxidants & redox signaling. 2007;9:855–863. - PubMed
-
- Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L'Abbate A, Kappas A, Abraham NG. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508–515. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01HL-125445/HL/NHLBI NIH HHS/United States
- HL106365/HL/NHLBI NIH HHS/United States
- P01 HL051971/HL/NHLBI NIH HHS/United States
- T32 HL105324/HL/NHLBI NIH HHS/United States
- L32 MD009154/MD/NIMHD NIH HHS/United States
- HL088421/HL/NHLBI NIH HHS/United States
- R25 HL106365/HL/NHLBI NIH HHS/United States
- P20 GM104357/GM/NIGMS NIH HHS/United States
- R01 HL088421/HL/NHLBI NIH HHS/United States
- K01 HL125445/HL/NHLBI NIH HHS/United States
- P20GM-104357/GM/NIGMS NIH HHS/United States
- 1T32HL105324/HL/NHLBI NIH HHS/United States
- P01HL-051971/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

