GATA1 induces epithelial-mesenchymal transition in breast cancer cells through PAK5 oncogenic signaling
- PMID: 25726523
- PMCID: PMC4414194
- DOI: 10.18632/oncotarget.2999
GATA1 induces epithelial-mesenchymal transition in breast cancer cells through PAK5 oncogenic signaling
Abstract
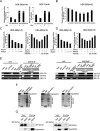
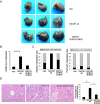
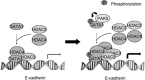
Epithelial-mesenchymal transition (EMT) is a key process in tumor metastatic cascade that is characterized by the loss of cell-cell junctions, resulting in the acquisition of migratory and invasive properties. E-cadherin is a major component of intercellular junctions and the reduction or loss of its expression is a hallmark of EMT. Transcription factor GATA1 has a critical anti-apoptotic role in breast cancer, but its function for metastasis has not been investigated. Here, we found that GATA1, as a novel E-cadherin repressor, promotes EMT in breast cancer cells. GATA1 binds to E-cadherin promoter, down-regulates E-cadherin expression, disrupts intercellular junction and promotes metastasis of breast cancer cell in vivo. Moreover, GATA1 is a new substrate of p21-activated kinase 5 (PAK5), which is phosphorylated on serine 161 and 187 (S161 and S187). GATA1 recruits HDAC3/4 to E-cadherin promoter, which is reduced by GATA1 S161A S187A mutant. These data indicate that phosphorylated GATA1 recruits more HDAC3/4 to promote transcriptional repression of E-cadherin, leading to the EMT of breast cancer cells. Our findings provide insights into the novel function of GATA1, contributing to a better understanding of the EMT, indicating that GATA1 and its phosphorylation may play an important role in the metastasis of breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
-
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. - PubMed
-
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

