Nuclear-encoded cytochrome c oxidase subunit 4 regulates BMI1 expression and determines proliferative capacity of high-grade gliomas
- PMID: 25726526
- PMCID: PMC4414193
- DOI: 10.18632/oncotarget.3015
Nuclear-encoded cytochrome c oxidase subunit 4 regulates BMI1 expression and determines proliferative capacity of high-grade gliomas
Abstract
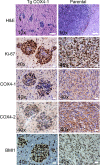
Nuclear-encoded cytochrome c oxidase subunit 4 (COX4) is a key regulatory subunit of mammalian cytochrome c oxidase, and recent studies have demonstrated that COX4 isoform 1 (COX4-1) could have a role in glioma chemoresistance. The Polycomb complex protein BMI1 is a stem cell regulatory gene implicated in the pathogenesis of many aggressive cancers, including glioma. This study sought to determine if COX4 regulates BMI1 and modulates tumor cell proliferation. Using The Cancer Genome Atlas database and a retrospective data set from patients with glioblastoma multiforme, we found that BMI1 expression levels positively correlated with COX4-1 expression and overall survival. Whereas COX4-1 promoted cell growth by increasing BMI1 expression, COX4-2 inhibited cell growth even in cells overexpressing BMI1. We also demonstrate that COX4-1 attenuates mitochondrial reactive oxygen species (ROS) production, which is required for COX4-1-mediated effects on BMI1 expression and cell proliferation. Notably, mice bearing COX4-1-expressing glioma cell xenografts quickly developed invasive tumors characterized by the presence of multiple lesions positive for Ki-67, BMI1, and COX4-1, whereas mice bearing COX4-2-expressing xenografts rarely developed tumors by this point. COX4-1 also promoted the self-renewal of glioma stem-like cells, consistent with the reported role of BMI1 in stem cell growth. Taken together, these findings identify a novel COX4-1-mitochondrial ROS axis, in which differential expression of COX4 isoforms regulates mitochondrial ROS production and controls BMI1 expression.
Figures






References
-
- Grossman LI, Lomax MI. Nuclear genes for cytochrome c oxidase. Biochimica et biophysica acta. 1997;1352:174–192. - PubMed
-
- Kadenbach B, Huttemann M, Arnold S, Lee I, Bender E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free radical biology & medicine. 2000;29:211–221. - PubMed
-
- Oliva CR, Nozell SE, Diers A, McClugage SG, 3rd, Sarkaria JN, Markert JM, Darley-Usmar VM, Bailey SM, Gillespie GY, Landar A, Griguer CE. Acquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chain. The Journal of biological chemistry. 2010;285:39759–39767. - PMC - PubMed
-
- Campian JL, Gao X, Qian M, Eaton JW. Cytochrome C oxidase activity and oxygen tolerance. The Journal of biological chemistry. 2007;282:12430–12438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

