Enantioselective desymmetrization of prochiral cyclohexanones by organocatalytic intramolecular Michael additions to α,β-unsaturated esters
- PMID: 25727215
- PMCID: PMC4678487
- DOI: 10.1002/anie.201411924
Enantioselective desymmetrization of prochiral cyclohexanones by organocatalytic intramolecular Michael additions to α,β-unsaturated esters
Abstract
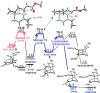
A new catalytic asymmetric desymmetrization reaction for the synthesis of enantioenriched derivatives of 2-azabicyclo[3.3.1]nonane, a key motif common to many alkaloids, has been developed. Employing a cyclohexanediamine-derived primary amine organocatalyst, a range of prochiral cyclohexanone derivatives possessing an α,β-unsaturated ester moiety linked to the 4-position afforded the bicyclic products, which possess three stereogenic centers, as single diastereoisomers in high enantioselectivity (83-99% ee) and in good yields (60-90%). Calculations revealed that stepwise C-C bond formation and proton transfer via a chair-shaped transition state dictate the exclusive endo selectivity and enabled the development of a highly enantioselective primary amine catalyst.
Keywords: Michael addition; desymmetrization; enamine catalysis; organocatalysis; quantum-chemical calculations.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



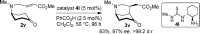
Similar articles
-
Enantioselective Michael Addition/Cyclization/Desymmetrization Sequence of Prochiral Cyclic Hemiacetals and Nitroolefins: Synthesis of Chiral Oxygen-Bridged Bicyclic Compounds.Org Lett. 2022 Dec 23;24(50):9254-9258. doi: 10.1021/acs.orglett.2c03815. Epub 2022 Dec 13. Org Lett. 2022. PMID: 36512320
-
Role of Cinchona Alkaloids in the Enantio- and Diastereoselective Synthesis of Axially Chiral Compounds.Acc Chem Res. 2022 Dec 20;55(24):3551-3571. doi: 10.1021/acs.accounts.2c00515. Epub 2022 Dec 7. Acc Chem Res. 2022. PMID: 36475607 Free PMC article.
-
Functionalized chiral ionic liquid catalyzed enantioselective desymmetrizations of prochiral ketones via asymmetric Michael addition reaction.J Org Chem. 2007 Nov 23;72(24):9350-2. doi: 10.1021/jo7020357. Epub 2007 Nov 2. J Org Chem. 2007. PMID: 17975933
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
-
[Development of Organocatalytic Reactions for the Synthesis of Natural Products and Pharmaceuticals].Yakugaku Zasshi. 2024;144(8):791-798. doi: 10.1248/yakushi.24-00095. Yakugaku Zasshi. 2024. PMID: 39085055 Review. Japanese.
Cited by
-
Quantum chemical calculations for reaction prediction in the development of synthetic methodologies.Chem Sci. 2023 Sep 30;14(42):11601-11616. doi: 10.1039/d3sc03319h. eCollection 2023 Nov 1. Chem Sci. 2023. PMID: 37920348 Free PMC article. Review.
-
OSCAR: an extensive repository of chemically and functionally diverse organocatalysts.Chem Sci. 2022 Oct 25;13(46):13782-13794. doi: 10.1039/d2sc04251g. eCollection 2022 Nov 30. Chem Sci. 2022. PMID: 36544722 Free PMC article.
-
Site-Selective Reaction of Enaminones and Enamine Esters for the Synthesis of Novel Diverse Morphan Derivatives.ACS Omega. 2018 Jun 4;3(6):5994-6005. doi: 10.1021/acsomega.8b00726. eCollection 2018 Jun 30. ACS Omega. 2018. PMID: 31458790 Free PMC article.
-
A New Organocatalytic Desymmetrization Reaction Enables the Enantioselective Total Synthesis of Madangamine E.J Am Chem Soc. 2022 Jan 26;144(3):1407-1415. doi: 10.1021/jacs.1c12040. Epub 2022 Jan 17. J Am Chem Soc. 2022. PMID: 35037758 Free PMC article.
-
Dual catalytic enantioselective desymmetrization of allene-tethered cyclohexanones.Chem Sci. 2020 Jun 24;11(28):7444-7450. doi: 10.1039/d0sc02878a. Chem Sci. 2020. PMID: 34123026 Free PMC article.
References
-
- For a review on natural products that contain a morphan core and strategies addressing their synthesis, see:
-
- Bonjoch J, Diaba F, Bradshaw B. Synthesis. 2011:993. for a recent synthesis of daphenylline, see:
-
- Lu Z, Li Y, Deng J, Li A. Nat. Chem. 2013;5:679. for a recent synthesis of (+)-madangamine D, see: - PubMed
-
- Ballette R, Pérez M, Proto S, Amat M, Bosch J. Angew. Chem. Int. Ed. 2014;53:6202. - PubMed
- Angew. Chem. 2014;126 for a recent review on strychnine, see:
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

