Innate memory T cells
- PMID: 25727290
- PMCID: PMC4670568
- DOI: 10.1016/bs.ai.2014.12.001
Innate memory T cells
Abstract
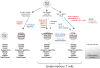
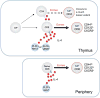
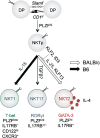
Memory T cells are usually considered to be a feature of a successful immune response against a foreign antigen, and such cells can mediate potent immunity. However, in mice, alternative pathways have been described, through which naïve T cells can acquire the characteristics and functions of memory T cells without encountering specific foreign antigen or the typical signals required for conventional T cell differentiation. Such cells reflect a response to the internal rather the external environment, and hence such cells are called innate memory T cells. In this review, we describe how innate memory subsets were identified, the signals that induce their generation and their functional properties and potential role in the normal immune response. The existence of innate memory T cells in mice raises questions about whether parallel populations exist in humans, and we discuss the evidence for such populations during human T cell development and differentiation.
Keywords: CD8 T cells; Cytokines; Homeostasis; Immune memory; NKT cells.
© 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

