Characterization of CD22 expression in acute lymphoblastic leukemia
- PMID: 25728039
- PMCID: PMC4405453
- DOI: 10.1002/pbc.25410
Characterization of CD22 expression in acute lymphoblastic leukemia
Abstract
Background: CD22 is a B-lineage differentiation antigen that has emerged as a leading therapeutic target in acute lymphoblastic leukemia (ALL).
Procedure: Properties of CD22 expression relevant to therapeutic targeting were characterized in primary samples obtained from children and young adults with relapsed and chemotherapy refractory B-precursor (pre-B) ALL.
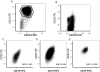
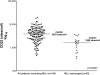
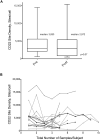
Results: CD22 expression was demonstrated in all subjects (n = 163) with detection on at least 90% of blasts in 155 cases. Median antigen site density of surface CD22 was 3,470 sites/cell (range 349-19,653, n = 160). Blasts from patients with known 11q23 (MLL) rearrangement had lower site density (median 1,590 sites/cell, range 349-3,624, n = 20 versus 3,853 sites/cell, range 451-19,653, n = 140; P = <0.0001) and 6 of 21 cases had sub-populations of blasts lacking CD22 expression (22%-82% CD22 +). CD22 expression was maintained in serial studies of 73 subjects, including those treated with anti-CD22 targeted therapy. The levels of soluble CD22 in blood and marrow by ELISA were low and not expected to influence the pharmacokinetics of anti-CD22 directed agents.
Conclusions: These characteristics make CD22 an excellent potential therapeutic target in patients with relapsed and chemotherapy-refractory ALL, although cases with MLL rearrangement require close study to exclude the presence of a CD22-negative blast population.
Keywords: CD22; acute lymphoblastic leukemia; monoclonal antibody; relapse.
Published 2015. This article is a U.S. Government work and is in the public domain in the USA.
Figures

References
-
- Gloeckler Ries L, Mortality CC. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. In: Reis LAGSM, Gurney JG, editors. SEER Program. National Cancer Institute; Bethesda: 1999. pp. 165–170. NIH Pub No 99-4649.
-
- Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E, Seibel NL, Twist CJ, Eckroth E, Sposto R, Gaynon PS, Loh ML. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28(4):648–654. doi:JCO.2009.22.2950 [pii] 10.1200/JCO.2009.22.2950. - PMC - PubMed
-
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. - DOI - PubMed
-
- Law CL, Aruffo A, Chandran KA, Doty RT, Clark EA. Ig domains 1 and 2 of murine CD22 constitute the ligand-binding domain and bind multiple sialylated ligands expressed on B and T cells. J Immunol. 1995;155(7):3368–3376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

