Single-unit analysis of somatosensory processing in the core auditory cortex of hearing ferrets
- PMID: 25728185
- PMCID: PMC4347953
- DOI: 10.1111/ejn.12828
Single-unit analysis of somatosensory processing in the core auditory cortex of hearing ferrets
Abstract
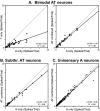
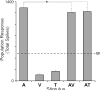
The recent findings in several species that the primary auditory cortex processes non-auditory information have largely overlooked the possibility of somatosensory effects. Therefore, the present investigation examined the core auditory cortices (anterior auditory field and primary auditory cortex) for tactile responsivity. Multiple single-unit recordings from anesthetised ferret cortex yielded histologically verified neurons (n = 311) tested with electronically controlled auditory, visual and tactile stimuli, and their combinations. Of the auditory neurons tested, a small proportion (17%) was influenced by visual cues, but a somewhat larger number (23%) was affected by tactile stimulation. Tactile effects rarely occurred alone and spiking responses were observed in bimodal auditory-tactile neurons. However, the broadest tactile effect that was observed, which occurred in all neuron types, was that of suppression of the response to a concurrent auditory cue. The presence of tactile effects in the core auditory cortices was supported by a substantial anatomical projection from the rostral suprasylvian sulcal somatosensory area. Collectively, these results demonstrate that crossmodal effects in the auditory cortex are not exclusively visual and that somatosensation plays a significant role in modulation of acoustic processing, and indicate that crossmodal plasticity following deafness may unmask these existing non-auditory functions.
Keywords: auditory; crossmodal; deaf; plasticity; tactile; vision.
© 2015 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures



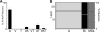


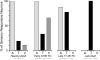
References
-
- Aitkin LM, Kenyon CE, Philpott P. The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. J. Comp. Neurol. 1981;196:25–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

