Old, new, and widely true: The bacteriophage T4 DNA packaging mechanism
- PMID: 25728298
- PMCID: PMC4424107
- DOI: 10.1016/j.virol.2015.01.015
Old, new, and widely true: The bacteriophage T4 DNA packaging mechanism
Abstract
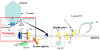
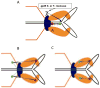
DNA packaging into empty viral procapsids by ATP-driven motor proteins applies widely among viruses. Recent fluorescence studies of phage T4 reveal: 1) the small terminase subunit (TerS) synapses pac homologs by a twin ring mechanism to gauge DNA maturation and allow packaging by the large terminase subunit (TerL); 2) translocation of linear DNA is efficient by TerL acting alone; expansion of the procapsid is controlled by the portal-terminase assembly; 3) both ends of the packaged DNA are held at the portal, showing a loop of DNA is packaged; 4) transient spring-like compression of B form to A form-like DNA accompanies translocation; 5) the C-terminal domain of TerL is docked to the portal and moves toward it when stalled; 6) a portal bound resolvase can release stalled Y-DNA compression and allow translocation in vitro; and 7) ATP powered translocation on A form dsDNA is supported by recent hexameric helicase studies.
Keywords: A form DNA; DNA motors; DNA packaging; Helicase; Pac site; Terminase.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
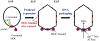


References
-
- Baumann RG, Mullaney J, Black LW. Portal fusion protein constraints on function in DNA packaging of bacteriophage T4. Mol Microbiol. 2006;61:16–32. - PubMed
-
- Black LW. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. - PubMed
-
- Black LW. DNA packaging and cutting by phage terminases: control in phage T4 by a synaptic mechanism. Bioessays. 1995;17:1025–1030. - PubMed
-
- Black LW, Peng G. Mechanistic coupling of bacteriophage T4 DNA packaging to components of the replication-dependent late transcription machinery. J Biol Chem. 2006;281:25635–25643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

