Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes
- PMID: 25728481
- PMCID: PMC4464961
- DOI: 10.1111/xen.12161
Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes
Abstract
Background: Simultaneous inactivation of pig GGTA1 and CMAH genes eliminates carbohydrate xenoantigens recognized by human antibodies. The β4GalNT2 glycosyltransferase may also synthesize xenoantigens. To further characterize glycan-based species incompatibilities, we examined human and non-human primate antibody binding to cells derived from genetically modified pigs lacking these carbohydrate-modifying genes.
Methods: The Cas9 endonuclease and gRNA were used to create pigs lacking GGTA1, GGTA1/CMAH, or GGTA1/CMAH/β4GalNT2 genes. Peripheral blood mononuclear cells were isolated from these animals and examined for binding to IgM and IgG from humans, rhesus macaques, and baboons.
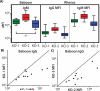
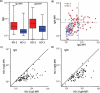
Results: Cells from GGTA1/CMAH/β4GalNT2 deficient pigs exhibited reduced human IgM and IgG binding compared to cells lacking both GGTA1 and CMAH. Non-human primate antibody reactivity with cells from the various pigs exhibited a slightly different pattern of reactivity than that seen in humans. Simultaneous inactivation of the GGTA1 and CMAH genes increased non-human primate antibody binding compared to cells lacking either GGTA1 only or to those deficient in GGTA1/CMAH/β4GalNT2.
Conclusions: Inactivation of the β4GalNT2 gene reduces human and non-human primate antibody binding resulting in diminished porcine xenoantigenicity. The increased humoral immunity of non-human primates toward GGTA1-/CMAH-deficient cells compared to pigs lacking either GGTA1 or GGTA1/CMAH/β4GalNT2 highlights the complexities of carbohydrate xenoantigens and suggests potential limitations of the non-human primate model for examining some genetic modifications. The progressive reduction of swine xenoantigens recognized by human immunoglobulin through inactivation of pig GGTA1/CMAH/β4GalNT2 genes demonstrates that the antibody barrier to xenotransplantation can be minimized by genetic engineering.
Keywords: CRISPR; Cas9; antibody; genetic engineering; primate; swine; xenoantigen; β4GalNT2.
© 2015 The Authors. Xenotransplantation Published by John Wiley & Sons Ltd.
Figures



References
-
- Galili U. Discovery of the natural anti-Gal antibody and its past and future relevance to medicine. Xenotransplantation. 2013;20(3):138–147. - PubMed
-
- Salama A, Evanno G, Harb J, Soulillou J. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2014 DOI: 10.1111/xen.12142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

