Participation of proteasome-ubiquitin protein degradation in autophagy and the activation of AMP-activated protein kinase
- PMID: 25728513
- PMCID: PMC4380640
- DOI: 10.1016/j.cellsig.2015.02.024
Participation of proteasome-ubiquitin protein degradation in autophagy and the activation of AMP-activated protein kinase
Abstract
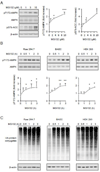
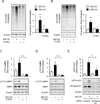
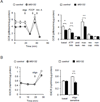
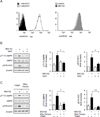
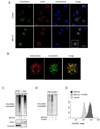
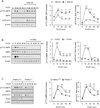
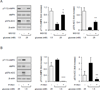
Although activation of the AMP-activated protein kinase (AMPK) as well as of ubiquitin/proteasome degradative pathways play an essential role in the preservation of metabolic homeostasis, little is known concerning interactions between protein turnover and AMPK activity. In the present studies, we found that inhibition of the 26S proteasome resulted in rapid activation of AMPK in macrophages, epithelial and endothelial cells. This was associated with increased levels of non-degraded Ub-protein conjugates, in both cytosolic and mitochondrial fractions. Selective inhibitors of ubiquitination or siRNA-dependent knockdown of Ub-ligase E1 diminished AMPK activation in cells treated with MG132, a 26S proteasome inhibitor. In addition to inhibition of AMPK activation by Ub-ligase E1 inhibitors, deficiency in Park2 mitochondria-associated Ub-ligase E3 also reduced AMPK activation upon dissipation of mitochondrial membrane potential (Δψm). Accumulation of Ub-proteins was correlated with decreases in cellular bioenergetics, including mitochondria oxidative phosphorylation, and an increase in ROS formation. Antioxidants, such as N-acetyl-L-cysteine or mitochondria-targeted MitoTEMPO, effectively diminished MG132-induced AMPK activation. Glucose-dependent regulation of AMPK or AMPK-mediated autophagy was modulated by alterations in intracellular levels of Ub-protein conjugates. Our results indicate that accumulation of ubiquitinated proteins alter cellular bioenergetics and redox status, leading to AMPK activation.
Keywords: AMP kinase; Autophagy; Bioenergetics; PARK2; Proteasome; Ubiquitination.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declare no conflict of interest
Figures


Similar articles
-
The GID ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan.Autophagy. 2020 Sep;16(9):1618-1634. doi: 10.1080/15548627.2019.1695399. Epub 2019 Dec 3. Autophagy. 2020. PMID: 31795790 Free PMC article.
-
Inhibition of proteasome reveals basal mitochondrial ubiquitination.J Proteomics. 2020 Oct 30;229:103949. doi: 10.1016/j.jprot.2020.103949. Epub 2020 Aug 31. J Proteomics. 2020. PMID: 32882436
-
Suppression of autophagy during mitosis via CUL4-RING ubiquitin ligases-mediated WIPI2 polyubiquitination and proteasomal degradation.Autophagy. 2019 Nov;15(11):1917-1934. doi: 10.1080/15548627.2019.1596484. Epub 2019 Mar 30. Autophagy. 2019. PMID: 30898011 Free PMC article.
-
Ubiquitin-proteasome system and mitochondria - reciprocity.Biochim Biophys Acta. 2011 Feb;1809(2):80-7. doi: 10.1016/j.bbagrm.2010.07.005. Epub 2010 Jul 30. Biochim Biophys Acta. 2011. PMID: 20674813 Review.
-
The Roles of Ubiquitin-Binding Protein Shuttles in the Degradative Fate of Ubiquitinated Proteins in the Ubiquitin-Proteasome System and Autophagy.Cells. 2019 Jan 10;8(1):40. doi: 10.3390/cells8010040. Cells. 2019. PMID: 30634694 Free PMC article. Review.
Cited by
-
USP8 inhibitor-induced DNA damage activates cell cycle arrest, apoptosis, and autophagy in esophageal squamous cell carcinoma.Cell Biol Toxicol. 2023 Oct;39(5):2011-2032. doi: 10.1007/s10565-021-09686-x. Epub 2022 Jan 13. Cell Biol Toxicol. 2023. PMID: 35022897
-
N-cadherin coordinates AMP kinase-mediated lung vascular repair.Am J Physiol Lung Cell Mol Physiol. 2016 Jan 1;310(1):L71-85. doi: 10.1152/ajplung.00227.2015. Epub 2015 Nov 6. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26545901 Free PMC article.
-
Genome-wide RNAi Screen for Fat Regulatory Genes in C. elegans Identifies a Proteostasis-AMPK Axis Critical for Starvation Survival.Cell Rep. 2017 Jul 18;20(3):627-640. doi: 10.1016/j.celrep.2017.06.068. Cell Rep. 2017. PMID: 28723566 Free PMC article.
-
The role of deubiquitinases in cardiac disease.Expert Rev Mol Med. 2024 Mar 25;26:e3. doi: 10.1017/erm.2024.2. Expert Rev Mol Med. 2024. PMID: 38525836 Free PMC article. Review.
-
Emerging Roles of Non-proteolytic Ubiquitination in Tumorigenesis.Front Cell Dev Biol. 2022 Jul 6;10:944460. doi: 10.3389/fcell.2022.944460. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35874839 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

