Helicobacter pylori Eradication in Patients with Immune Thrombocytopenic Purpura: A Review and the Role of Biogeography
- PMID: 25728540
- PMCID: PMC4506733
- DOI: 10.1111/hel.12200
Helicobacter pylori Eradication in Patients with Immune Thrombocytopenic Purpura: A Review and the Role of Biogeography
Abstract
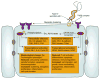
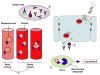
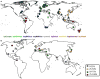
Idiopathic thrombocytopenic purpura (ITP) is typically a diagnosis of exclusion, assigned by clinicians after ruling out other identifiable etiologies. Since a report by Gasbarrini et al. in 1998, an accumulating body of evidence has proposed a pathophysiological link between ITP and chronic Helicobacter pylori (H. pylori) infection. Clinical reports have described a spontaneous resolution of ITP symptoms in about 50% of chronic ITP patients following empirical treatment of H. pylori infection, but response appears to be geography dependent. Studies have also documented that ITP patients in East Asian countries are more likely to express positive antibody titers against H. pylori-specific cytotoxic-associated gene A (CagA), a virulence factor that is associated with an increased risk for gastric diseases including carcinoma. While a definitive mechanism by which H. pylori may induce thrombocytopenia remains elusive, proposed pathways include molecular mimicry of CagA by host autoantibodies against platelet surface glycoproteins, as well as perturbations in the phagocytic activity of monocytes. Traditional treatments of ITP have been largely empirical, involving the use of immunosuppressive agents and immunoglobulin therapy. However, based on the findings of clinical reports emerging over the past 20 years, health organizations around the world increasingly suggest the detection and eradication of H. pylori as a treatment for ITP. Elucidating the exact molecular mechanisms of platelet activation in H. pylori-positive ITP patients, while considering biogeographical differences in response rates, could offer insight into how best to use clinical H. pylori eradication to treat ITP, but will require well-designed studies to confirm the suggested causative relationship between bacterial infection and an autoimmune disease state.
Keywords: CagA; Helicobacter; biogeography; immune-mediated; infectious; thrombocytopenia.
© 2015 John Wiley & Sons Ltd.
Conflict of interest statement
Figures

Similar articles
-
Does H. Pylori infection play a role in idiopathic thrombocytopenic purpura and in other autoimmune diseases?Am J Gastroenterol. 2005 Jun;100(6):1271-3. doi: 10.1111/j.1572-0241.2005.50224.x. Am J Gastroenterol. 2005. PMID: 15929756
-
Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms.World J Gastroenterol. 2014 Jan 21;20(3):714-23. doi: 10.3748/wjg.v20.i3.714. World J Gastroenterol. 2014. PMID: 24574745 Free PMC article. Review.
-
Effect of Helicobacter pylori eradication in patients with chronic idiopathic thrombocytopenic purpura-a randomized controlled trial.Am J Gastroenterol. 2005 Jun;100(6):1265-70. doi: 10.1111/j.1572-0241.2005.41641.x. Am J Gastroenterol. 2005. PMID: 15929755 Clinical Trial.
-
Characteristics of Helicobacter pylori-induced gastritis and the effect of H. pylori eradication in patients with chronic idiopathic thrombocytopenic purpura.Helicobacter. 2004 Oct;9(5):443-52. doi: 10.1111/j.1083-4389.2004.00261.x. Helicobacter. 2004. PMID: 15361084
-
Helicobacter pylori infection and idiopathic thrombocytopenic purpura.Int J Hematol. 2005 Feb;81(2):113-8. doi: 10.1532/ijh97.04161. Int J Hematol. 2005. PMID: 15765778 Review.
Cited by
-
Autoimmune Hepatitis with Concomitant Idiopathic Thrombocytopenic Purpura Diagnosed by Transjugular Liver Biopsy.Case Reports Hepatol. 2018 Dec 9;2018:5305691. doi: 10.1155/2018/5305691. eCollection 2018. Case Reports Hepatol. 2018. PMID: 30631611 Free PMC article.
-
Old and New Aspects of H. pylori-Associated Inflammation and Gastric Cancer.Children (Basel). 2022 Jul 20;9(7):1083. doi: 10.3390/children9071083. Children (Basel). 2022. PMID: 35884067 Free PMC article. Review.
-
Helicobacter pylori induced Immune Thrombocytopenic Purpura and perspective role of Helicobacter pylori eradication therapy for treating Immune Thrombocytopenic Purpura.AIMS Microbiol. 2021 Sep 2;7(3):284-303. doi: 10.3934/microbiol.2021018. eCollection 2021. AIMS Microbiol. 2021. PMID: 34708173 Free PMC article. Review.
-
Helicobacter pylori metabolites exacerbate gastritis through C-type lectin receptors.J Exp Med. 2021 Jan 4;218(1):e20200815. doi: 10.1084/jem.20200815. J Exp Med. 2021. PMID: 32991669 Free PMC article.
-
Successful treatment of coexisting membranous nephropathy and immune thrombocytopenia by eradicating gastric Helicobacter pylori infection: a case report.CEN Case Rep. 2024 Apr;13(2):98-103. doi: 10.1007/s13730-023-00805-7. Epub 2023 Jul 8. CEN Case Rep. 2024. PMID: 37421572 Free PMC article.
References
-
- Rothenbacher D, Brenner H. Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: recent developments and future implications. Microbes Infect. 2003;5:693–703. - PubMed
-
- Rothenbacher D, Winkler M, Gonser T, Adler G, Brenner H. Role of infected parents in transmission of Helicobacter pylori to their children. Pediatr Infect Dis J. 2002;21:674–9. - PubMed
-
- Frenck RW, Jr, Clemens J. Helicobacter in the developing world. Microbes Infect. 2003;5:705–13. - PubMed
-
- Malaty HM, El-Kasabany A, Graham DY, Miller CC, Reddy SG, Srinivasan SR, Yamaoka Y, Berenson GS. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931–5. - PubMed
-
- Parsonnet J, Shmuely H, Haggerty T. Fecal and oral shedding of Helicobacter pylori from healthy infected adults. JAMA. 1999;282:2240–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

