TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model
- PMID: 25728668
- PMCID: PMC4477963
- DOI: 10.1016/j.cell.2015.01.049
TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model
Abstract
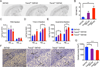
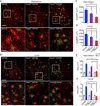
Triggering receptor expressed on myeloid cells 2 (TREM2) is a microglial surface receptor that triggers intracellular protein tyrosine phosphorylation. Recent genome-wide association studies have shown that a rare R47H mutation of TREM2 correlates with a substantial increase in the risk of developing Alzheimer's disease (AD). To address the basis for this genetic association, we studied TREM2 deficiency in the 5XFAD mouse model of AD. We found that TREM2 deficiency and haploinsufficiency augment β-amyloid (Aβ) accumulation due to a dysfunctional response of microglia, which fail to cluster around Aβ plaques and become apoptotic. We further demonstrate that TREM2 senses a broad array of anionic and zwitterionic lipids known to associate with fibrillar Aβ in lipid membranes and to be exposed on the surface of damaged neurons. Remarkably, the R47H mutation impairs TREM2 detection of lipid ligands. Thus, TREM2 detects damage-associated lipid patterns associated with neurodegeneration, sustaining the microglial response to Aβ accumulation.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




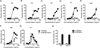
Comment in
-
TREM2 enables amyloid β clearance by microglia.Cell Res. 2015 May;25(5):535-6. doi: 10.1038/cr.2015.37. Epub 2015 Mar 31. Cell Res. 2015. PMID: 25828532 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

