Tuning cytokine receptor signaling by re-orienting dimer geometry with surrogate ligands
- PMID: 25728669
- PMCID: PMC4766813
- DOI: 10.1016/j.cell.2015.02.011
Tuning cytokine receptor signaling by re-orienting dimer geometry with surrogate ligands
Abstract
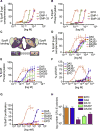
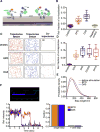
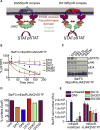
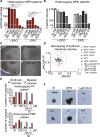
Most cell-surface receptors for cytokines and growth factors signal as dimers, but it is unclear whether remodeling receptor dimer topology is a viable strategy to "tune" signaling output. We utilized diabodies (DA) as surrogate ligands in a prototypical dimeric receptor-ligand system, the cytokine Erythropoietin (EPO) and its receptor (EpoR), to dimerize EpoR ectodomains in non-native architectures. Diabody-induced signaling amplitudes varied from full to minimal agonism, and structures of these DA/EpoR complexes differed in EpoR dimer orientation and proximity. Diabodies also elicited biased or differential activation of signaling pathways and gene expression profiles compared to EPO. Non-signaling diabodies inhibited proliferation of erythroid precursors from patients with a myeloproliferative neoplasm due to a constitutively active JAK2V617F mutation. Thus, intracellular oncogenic mutations causing ligand-independent receptor activation can be counteracted by extracellular ligands that re-orient receptors into inactive dimer topologies. This approach has broad applications for tuning signaling output for many dimeric receptor systems.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Active conformation of the erythropoietin receptor: random and cysteine-scanning mutagenesis of the extracellular juxtamembrane and transmembrane domains.J Biol Chem. 2006 Mar 17;281(11):7002-11. doi: 10.1074/jbc.M512638200. Epub 2006 Jan 12. J Biol Chem. 2006. PMID: 16414957
-
Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation.Science. 1999 Feb 12;283(5404):987-90. doi: 10.1126/science.283.5404.987. Science. 1999. PMID: 9974392
-
Active and inactive orientations of the transmembrane and cytosolic domains of the erythropoietin receptor dimer.Mol Cell. 2003 Nov;12(5):1239-50. doi: 10.1016/s1097-2765(03)00389-7. Mol Cell. 2003. PMID: 14636581
-
Activation of erythropoietin signaling by receptor dimerization.Int J Biochem Cell Biol. 1999 Oct;31(10):1075-88. doi: 10.1016/s1357-2725(99)00075-8. Int J Biochem Cell Biol. 1999. PMID: 10582340 Review.
-
The structure, organization, activation and plasticity of the erythropoietin receptor.Curr Opin Struct Biol. 1999 Dec;9(6):696-704. doi: 10.1016/s0959-440x(99)00032-9. Curr Opin Struct Biol. 1999. PMID: 10607675 Review.
Cited by
-
JAK inhibitors and COVID-19.J Immunother Cancer. 2022 Apr;10(4):e002838. doi: 10.1136/jitc-2021-002838. J Immunother Cancer. 2022. PMID: 35459733 Free PMC article. Review.
-
Crystal structures of mono- and bi-specific diabodies and reduction of their structural flexibility by introduction of disulfide bridges at the Fv interface.Sci Rep. 2016 Sep 29;6:34515. doi: 10.1038/srep34515. Sci Rep. 2016. PMID: 27682821 Free PMC article.
-
Emerging principles of cytokine pharmacology and therapeutics.Nat Rev Drug Discov. 2023 Jan;22(1):21-37. doi: 10.1038/s41573-022-00557-6. Epub 2022 Sep 21. Nat Rev Drug Discov. 2023. PMID: 36131080 Free PMC article. Review.
-
Determinants of Ligand Specificity and Functional Plasticity in Type I Interferon Signaling.Front Immunol. 2021 Oct 7;12:748423. doi: 10.3389/fimmu.2021.748423. eCollection 2021. Front Immunol. 2021. PMID: 34691060 Free PMC article.
-
STAT5 as a Key Protein of Erythropoietin Signalization.Int J Mol Sci. 2021 Jul 1;22(13):7109. doi: 10.3390/ijms22137109. Int J Mol Sci. 2021. PMID: 34281163 Free PMC article. Review.
References
-
- Ballinger MD, Wells JA. Will any dimer do? Nat Struct Biol. 1998;5:938–940. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. - PubMed
-
- Bazan JF. Haemopoietic receptors and helical cytokines. Immunol Today. 1990;11:350–354. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

