De novo nonsense mutations in KAT6A, a lysine acetyl-transferase gene, cause a syndrome including microcephaly and global developmental delay
- PMID: 25728775
- PMCID: PMC4375619
- DOI: 10.1016/j.ajhg.2015.01.017
De novo nonsense mutations in KAT6A, a lysine acetyl-transferase gene, cause a syndrome including microcephaly and global developmental delay
Abstract
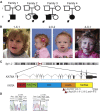
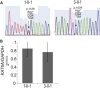
Chromatin remodeling through histone acetyltransferase (HAT) and histone deactylase (HDAC) enzymes affects fundamental cellular processes including the cell-cycle, cell differentiation, metabolism, and apoptosis. Nonsense mutations in genes that are involved in histone acetylation and deacetylation result in multiple congenital anomalies with most individuals displaying significant developmental delay, microcephaly and dysmorphism. Here, we report a syndrome caused by de novo heterozygous nonsense mutations in KAT6A (a.k.a., MOZ, MYST3) identified by clinical exome sequencing (CES) in four independent families. The same de novo nonsense mutation (c.3385C>T [p.Arg1129∗]) was observed in three individuals, and the fourth individual had a nearby de novo nonsense mutation (c.3070C>T [p.Arg1024∗]). Neither of these variants was present in 1,815 in-house exomes or in public databases. Common features among all four probands include primary microcephaly, global developmental delay including profound speech delay, and craniofacial dysmorphism, as well as more varied features such as feeding difficulties, cardiac defects, and ocular anomalies. We further demonstrate that KAT6A mutations result in dysregulation of H3K9 and H3K18 acetylation and altered P53 signaling. Through histone and non-histone acetylation, KAT6A affects multiple cellular processes and illustrates the complex role of acetylation in regulating development and disease.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Doenecke D. Chromatin dynamics from S-phase to mitosis: contributions of histone modifications. Cell Tissue Res. 2014;356:467–475. - PubMed
-
- Leung K.S., Cheng V.W., Mok S.W., Tsui S.K. The involvement of DNA methylation and histone modification on the epigenetic regulation of embryonic stem cells and induced pluripotent stem cells. Curr. Stem Cell Res. Ther. 2014;9:388–395. - PubMed
-
- Yang X.J., Ullah M. MOZ and MORF, two large MYSTic HATs in normal and cancer stem cells. Oncogene. 2007;26:5408–5419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

