Theophylline Pharmacokinetics in Foetal Sheep: Maternal Metabolic Capacity is the Principal Driver
- PMID: 25728792
- PMCID: PMC4750914
- DOI: 10.1111/bcpt.12395
Theophylline Pharmacokinetics in Foetal Sheep: Maternal Metabolic Capacity is the Principal Driver
Abstract
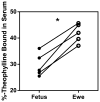
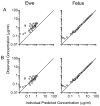
Understanding theophylline pharmacokinetics (PK) in the foetus is essential to prevent in utero toxicity and optimize prophylactic therapies. Previous studies in pregnancy have been obfuscated by maternal dosing and inadequate sampling in the foetus; both render modelling of foetal PK difficult. Six ewes carrying singleton foetuses received theophylline (60 mg) into the foetal jugular vein. Blood samples were drawn from the foetus and ewe over 36 hr. Serum concentrations were measured. Maternal and foetal pharmacokinetic parameters were estimated. Foetal non-compartmental pharmacokinetic parameters were as follows: half-life 7.37 ± 1.22 hr; volume of distribution 44.62 ± 11.45 L; area under the curve 14.82 ± 2.71 hr/(μg/mL); and clearance 4.15 ± 0.70 L/hr. Rapid theophylline distribution across the placenta was observed. Maternal non-compartmental pharmacokinetic parameters were as follows: half-life 6.54 ± 2.44 hr; volume of distribution 32.48 ± 9.99 L; area under the curve 16.28 ± 4.53 hr/(μg/mL); and clearance 3.69 ± 1.47 L/hr. Foetal and ewe serum concentration-time profiles were fit together into a 3-compartment population pharmacokinetic model, and parameters were as follows: central volume 1.38 ± 0.11 L; 2nd peripheral compartment volume 3.11 ± 0.29 L; 3rd peripheral compartment volume 60.14 ± 6.02 L; elimination clearance 9.89 ± 0.90 L/hr; distribution clearance between central and 2nd compartment 30.87 ± 2.31 L/hr; and distribution clearance between 2nd and 3rd compartments 13.89 ± 1.11 L/hr. Cytochrome P4501A expression was robust in maternal liver; negligible activities were observed in placenta, foetal liver and foetal kidney. In vitro protein binding of theophylline was 30% lower in foetal serum compared to maternal serum (29.7 ± 4.4 versus 42.0 ± 3.6%-bound). Free concentrations were lower in the foetus than in the ewe, suggesting active transport across placenta. In summary, foetal clearance of theophylline is attributable to rapid distribution into the maternal circulation across the placenta followed by greater maternal protein binding and metabolic activity.
© 2015 Nordic Association for the Publication of BCPT (former Nordic Pharmacological Society).
Figures



References
-
- Aden U. Methylxanthines during pregnancy and early postnatal life. Handb Exp Pharmacol. 2011;200:373–89. - PubMed
-
- Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol. 2011;200:33–91. - PubMed
-
- Tilley SL. Methylxanthines in asthma. Handb Exp Pharmacol. 2011;200:439–56. - PubMed
-
- Bissonnette JM, Hohimer AR, Chao CR, Knopp SJ, Notoroberto NF. Theophylline stimulates fetal breathing movements during hypoxia. Pediatr Res. 1990;28:83–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

