Pharmacokinetics, dynamics and toxicity of docetaxel: Why the Japanese dose differs from the Western dose
- PMID: 25728850
- PMCID: PMC4452149
- DOI: 10.1111/cas.12647
Pharmacokinetics, dynamics and toxicity of docetaxel: Why the Japanese dose differs from the Western dose
Abstract
Docetaxel (Taxotere(®)) has been one of the most important chemotherapeutic drugs for cancer treatment since 1996. Although a large number of clinical studies have been conducted in various cancer fields, there is a discrepancy in the standard dose between Japan and Western countries. This article reviews the pharmacokinetic, pharmacodynamic and toxicological profiles of docetaxel, and explains why there exists an ethnic difference in dose, and further discusses which direction we should go forward to solve this problem. The original recommended dose was 100 mg/m(2) every 3 weeks in US and European populations, while a Japanese phase I study suggested the recommended dose as 60 mg/m(2) every 3 weeks. A prospective population pharmacokinetic analysis of docetaxel conducted in both the USA/Europe and Japan, indicated an absence of ethnic difference in the pharmacokinetics. Both analyses demonstrated that docetaxel clearance is related to α1-acid glycoprotein level, hepatic function, age and body surface area. The relationship was observed between increasing docetaxel dose and increased tumor response rates across the dose range of 60 to 100 mg/m(2). The area under the serum concentration time curve (AUC) of docetaxel at the first cycle was significantly related to time to progression. Hematological toxicities were well correlated with the AUC of docetaxel, and severe hematological toxicities were more frequently observed in Japanese patients treated with 60 mg/m(2), compared to the US/European patients treated with 75-100 mg/m(2) dose. The Japanese population seems more susceptible to the toxicity of docetaxel. A docetaxel dose of 75 mg/m(2) is now standard not only in global trials but also in recent Japanese trials. Although the optimal dose of docetaxel is still unclear, we need to continue to seek the appropriate dose of docetaxel depending on patient status and the goals of chemotherapy.
Keywords: Docetaxel; ethnic difference; pharmacodynamics; pharmacokinetics; toxicity.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures
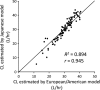
References
-
- Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83:288–91. - PubMed
-
- Taguchi T, Furue H, Niitani H, et al. Phase I clinical trial of RP 56976 (docetaxel) a new anticancer drug. Gan To Kagaku Ryoho. 1994;21:1997–2005. - PubMed
-
- Extra JM, Rousseau F, Bruno R, Clavel M, Le Bail N, Marty M. Phase I and pharmacokinetic study of Taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res. 1993;53:1037–42. - PubMed
-
- Burris H, Irvin R, Kuhn J, et al. Phase I clinical trial of taxotere administered as either a 2-hour or 6-hour intravenous infusion. J Clin Oncol. 1993;11:950–8. - PubMed
-
- Yamamoto N, Tamura T, Kamiya Y, Sekine I, Kunitoh H, Saijo N. Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J Clin Oncol. 2000;18:2301–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

